Abstract
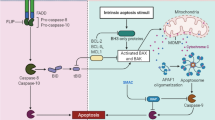
Cancer cell death is the utmost aim in cancer therapy. Anti-cancer agents can induce apoptosis, mitotic catastrophe, senescence, or autophagy through the production of free radicals and induction of DNA damage. However, cancer cells can acquire some new properties to adapt to anti-cancer agents. An increase in the incidence of apoptosis, mitotic catastrophe, senescence, and necrosis is in favor of overcoming tumor resistance to therapy. Although an increase in the autophagy process may help the survival of cancer cells, some studies indicated that stimulation of autophagy cell death may be useful for cancer therapy. Using some low toxic agents to amplify cancer cell death is interesting for the eradication of clonogenic cancer cells. Resveratrol (a polyphenol agent) may affect various signaling pathways related to cell death. It can induce death signals and also downregulate the expression of anti-apoptotic genes. Resveratrol has also been shown to modulate autophagy and induce mitotic catastrophe and senescence in some cancer cells. This review focuses on the important targets and mechanisms for the modulation of cancer cell death by resveratrol.




Similar content being viewed by others
References
Goradel NH, Mohajel N, Malekshahi ZV et al (2019) Oncolytic adenovirus: a tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol 234:8636–8646
Haddadi Gh RA, Ma M-S, Hosseinzadeh M, Fardid R, Najafi M et al (2017) Hesperidin as radioprotector against radiation-induced lung damage in rat: a histopathological study. J Med Phys 42:25–32
Rezaeyan AFR, Haddadi Gh, Ma T, Hosseinzadeh M, Najafi M et al (2016) Evaluating radioprotective effect of hesperidin on acute radiation damage in the lung tissue of rats. J Biomed Phys Eng 6:165–174
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
O’flanagan CH, Smith LA, Mcdonell SB et al (2017) When less may be more: calorie restriction and response to cancer therapy. BMC Med 15:1–9
Azmoonfar R, Amini P, Yahyapour R et al (2020) Mitigation of radiation-induced pneumonitis and lung fibrosis using alpha-lipoic acid and resveratrol. Anti-inflamm Anti-allergy Agents Med Chem 19:149–157
Mortezaee K, Goradel NH, Amini P et al (2019) NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr Mol Pharmacol 12:50–60
Van Der Valk M, Van Etten B, Marijnen C et al (2020) Compliance, acute toxicity and postoperative complications of short-course radiotherapy followed by chemotherapy and surgery for high-risk rectal cancer. Results of the randomized RAPIDO-trial. Eur J Surg Oncol 46:e20
Ji K, Sun X, Liu Y et al (2018) Regulation of apoptosis and radiation sensitization in lung cancer cells via the Sirt1/NF-κB/Smac pathway. Cell Physiol Biochem 48:304–316
Lhuillier C, Rudqvist N-P, Elemento O et al (2019) Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med 11:40
Schulz A, Meyer F, Dubrovska A et al (2019) Cancer stem cells and radioresistance: DNA repair and beyond. Cancers 11:862
Ashrafizadeh M, Farhood B, Musa AE et al (2020) The interactions and communications in tumor resistance to radiotherapy: therapy perspectives. Int Immunopharmacol 87:106807
Farhood B, Aliasgharzadeh A, Amini P et al (2019) Radiation-induced dual oxidase upregulation in rat heart tissues: protective effect of melatonin. Medicina (Kaunas, Lithuania) 55:317
Yahyapour R, Shabeeb D, Cheki M et al (2018) Radiation protection and mitigation by natural antioxidants and flavonoids: implications to radiotherapy and radiation disasters. Curr Mol Pharmacol 11:285–304
Mortezaee K (2020) Immune escape: a critical hallmark in solid tumors. Life Sci 258:118110
Najafi M, Mortezaee K, Majidpoor J (2019) Stromal reprogramming: a target for tumor therapy. Life Sci 239:117049
Ashrafizadeh M, Farhood B, Musa AE et al (2020) Damage-associated molecular patterns in tumor radiotherapy. Int Immunopharmacol 86:106761
Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90:127–156
Amini P, Ashrafizadeh M, Motevaseli E et al (2020) Mitigation of radiation-induced hematopoietic system injury by melatonin. Environ Toxicol 35:815–821
Nodooshan SJ, Amini P, Ashrafizadeh M et al (2020) Suberosin attenuates the proliferation of MCF-7 breast cancer cells in combination with radiotherapy or hyperthermi. Curr Drug Res Rev. https://doi.org/10.2174/2589977512666201228104528
Farhood B, Ashrafizadeh M, Hoseini-Ghahfarokhi M et al (2020) Targeting of cellular redox metabolism for mitigation of radiation injury. Life Sci 250:117570
Ashrafizadeh M, Farhood B, Musa AE et al (2020) Abscopal effect in radioimmunotherapy. Int Immunopharmacol 85:106663
Mortezaee K, Parwaie W, Motevaseli E et al (2019) Targets for improving tumor response to radiotherapy. Int Immunopharmacol 76:105847
Mortezaee K, Najafi M, Farhood B et al (2019) Genomic instability and carcinogenesis of heavy charged particles radiation: clinical and environmental implications. Medicina (Kaunas) 55:591
Farhood B, Hoseini-Ghahfarokhi M, Motevaseli E et al (2020) TGF-β in radiotherapy: mechanisms of tumor resistance and normal tissues injury. Pharmacol Res 155:104745
Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P et al (2020) Targets for protection and mitigation of radiation injury. Cell Mol Life Sci 77:3129–3159
Mortezaee K, Narmani A, Salehi M et al (2021) Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci 269:119020
Ashrafizadeh M, Zarrabi A, Samarghandian S et al (2020) PTEN: what we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol 881:173226
Ashrafizadeh M, Najafi M, Ang HL et al (2020) PTEN, a barrier for proliferation and metastasis of gastric cancer cells: from molecular pathways to targeting and regulation. Biomedicines 8:264
Presa N, Gomez-Larrauri A, Dominguez-Herrera A et al (2020) Novel signaling aspects of ceramide 1-phosphate. Biochim Biophys Acta (BBA) 1865:158630
Larsen BD, Sørensen CS (2017) The caspase-activated DN ase: apoptosis and beyond. FEBS J 284:1160–1170
D’arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Kretz A-L, Von Karstedt S, Hillenbrand A et al (2018) Should we keep walking along the trail for pancreatic cancer treatment? Revisiting TNF-related apoptosis-inducing ligand for anticancer therapy. Cancers 10:77
Cao K, Tait SW (2018) Apoptosis and cancer: force awakens, phantom menace, or both? Int Rev Cell Mol Biol 337:135–152
Jardim FR, De Rossi FT, Nascimento MX et al (2018) Resveratrol and brain mitochondria: a review. Mol Neurobiol 55:2085–2101
Galati S, Boni C, Gerra MC et al (2019) Autophagy: a player in response to oxidative stress and DNA damage. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5692958
Miller DR, Thorburn A (2021) Autophagy and organelle homeostasis in cancer. Dev Cell 56:906–918
Maiuri MC, Zalckvar E, Kimchi A et al (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Smith AG, Macleod KF (2019) Autophagy, cancer stem cells and drug resistance. J Pathol 247:708–718
Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci 19:3466
Ashrafizadeh M, Zarrabi A, Orouei S et al (2021) MicroRNA-mediated autophagy regulation in cancer therapy: the role in chemoresistance/chemosensitivity. Eur J Pharmacol 892:173660
Vitale I, Galluzzi L, Castedo M et al (2011) Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 12:385–392
Adjemian S, Oltean T, Martens S et al (2020) Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis 11:1–15
Prokhorova EA, Egorshina AY, Zhivotovsky B et al (2020) The DNA-damage response and nuclear events as regulators of nonapoptotic forms of cell death. Oncogene 39:1–16
Portugal J, Mansilla S, Bataller M (2010) Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des 16:69–78
Kobayashi D, Oike T, Shibata A et al (2017) Mitotic catastrophe is a putative mechanism underlying the weak correlation between sensitivity to carbon ions and cisplatin. Sci Rep 7:1–8
Mc Gee MM (2015) Targeting the mitotic catastrophe signaling pathway in cancer. Mediat Inflamm 2015:146282
Gewirtz DA, Holt SE, Elmore LW (2008) Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol 76:947–957
Wunderlich R, Ruehle P-F, Deloch L et al (2017) Interconnection between DNA damage, senescence, inflammation, and cancer. Front Biosci 22:348–369
Campisi J, Kim S-H, Lim C-S et al (2001) Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 36:1619–1637
Nardella C, Clohessy JG, Alimonti A et al (2011) Pro-senescence therapy for cancer treatment. Nat Rev Cancer 11:503–511
Acosta JC, Gil J (2012) Senescence: a new weapon for cancer therapy. Trends Cell Biol 22:211–219
Amaravadi RK, Thompson CB (2007) The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res 13:7271–7279
Salehi B, Mishra AP, Nigam M et al (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6:91
Ashrafizadeh M, Taeb S, Haghi-Aminjan H et al (2020) Resveratrol as an enhancer of apoptosis in cancer: a mechanistic review. Anti-cancer Agents Med Chem. https://doi.org/10.2174/1871520620666201020160348
Mortezaee K, Najafi M, Farhood B et al (2020) Resveratrol as an adjuvant for normal tissues protection and tumor sensitization. Curr Cancer Drug Targets 20:130–145
Ahmadi Z, Mohammadinejad R, Ashrafizadeh M (2019) Drug delivery systems for resveratrol, a non-flavonoid polyphenol: emerging evidence in last decades. J Drug Deliv Sci Technol 51:591–604
Ashrafizadeh M, Ahmadi Z, Farkhondeh T et al (2020) Resveratrol targeting the Wnt signaling pathway: a focus on therapeutic activities. J Cell Physiol 235:4135–4145
Ashrafizadeh M, Javanmardi S, Moradi-Ozarlou M et al (2020) Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy: an updated review on resveratrol. Biosci Rep. https://doi.org/10.1042/BSR20200257
Senthil Kumar C, Thangam R, Mary SA et al (2020) Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym 231:115682
Summerlin N, Soo E, Thakur S et al (2015) Resveratrol nanoformulations: challenges and opportunities. Int J Pharm 479:282–290
Amini P, Nodooshan SJ, Ashrafizadeh M et al (2020) Resveratrol induces apoptosis and attenuates proliferation of MCF-7 cells in combination with radiation and hyperthermia. Curr Mol Med. https://doi.org/10.2174/1566524020666200521080953
Zhang Y, Yang S, Yang Y et al (2019) Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect Agents Cancer 14:27
Chhabra G, Singh CK, Amiri D et al (2021) Recent advancements on immunomodulatory mechanisms of resveratrol in tumor microenvironment. Molecules (Basel, Switzerland) 26:1343
Chen Q, Ganapathy S, Singh KP et al (2010) Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE 5:e15288
Wang Z, Wu L, Tong S et al (2016) Resveratrol suppresses the epithelial-to-mesenchymal transition in PC-3 cells by down-regulating the PI3K/AKT signaling pathway. Anim Cells Syst 20:77–85
Chai R, Fu H, Zheng Z et al (2017) Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep 16:8037–8044
Jin X, Wei Y, Liu Y et al (2019) Resveratrol promotes sensitization to Doxorubicin by inhibiting epithelial–mesenchymal transition and modulating SIRT1/β-catenin signaling pathway in breast cancer. Cancer Med 8:1246–1257
Xu L, Botchway BO, Zhang S et al (2018) Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Front Neurosci 12:690
Mortezaee K, Najafi M, Farhood B et al (2019) NF-kappaB targeting for overcoming tumor resistance and normal tissues toxicity. J Cell Physiol 234:17187–17204
Kotha A, Sekharam M, Cilenti L et al (2006) Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther 5:621
Li D, Wang G, Jin G et al (2019) Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int J Mol Med 43:630–640
Baek SH, Ko J-H, Lee H et al (2016) Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 23:566–577
Ma X, Tian X, Huang X et al (2007) Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca 2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem 302:99–109
Sareen D, Darjatmoko SR, Albert DM et al (2007) Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol 72:1466–1475
Liu Z, Wu X, Lv J et al (2019) Resveratrol induces p53 in colorectal cancer through SET7/9. Oncol Lett 17:3783–3789
Tian Y, Song W, Li D et al (2019) Resveratrol as a natural regulator of autophagy for prevention and treatment of cancer. Onco Targets Ther 12:8601–8609
Kueck A, Opipari AW, Griffith KA et al (2007) Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol Oncol 107:450–457
Zhang J, Chiu J, Zhang H et al (2013) Autophagic cell death induced by resveratrol depends on the Ca(2+)/AMPK/mTOR pathway in A549 cells. Biochem Pharmacol 86:317–328
Chang C-H, Lee C-Y, Lu C-C et al (2017) Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol 50:873–882
Miki H, Uehara N, Kimura A et al (2012) Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int J Oncol 40:1020–1028
García-Zepeda SP, García-Villa E, Díaz-Chávez J et al (2013) Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur J Cancer Prev 22:577–584
Lang F, Qin Z, Li F et al (2015) Apoptotic cell death induced by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells. PLoS ONE 10:e0129196
Wang H, Peng Y, Wang J et al (2018) Effect of autophagy on the resveratrol-induced apoptosis of ovarian cancer SKOV3 cells. J Cell Biochem 120:7788–7793
Yu J, Parkhitko A, Henske EP (2011) Autophagy: an ‘Achilles’ heel of tumorigenesis in TSC and LAM. Autophagy 7:1400–1401
He X, Wang Y, Zhu J et al (2011) Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett 301:168–176
Alayev A, Sun Y, Snyder RB et al (2014) Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle (Georgetown, Tex.) 13:371–382
Filippi-Chiela EC, Thomé MP, Bueno e Silva MM et al (2013) Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer 13:147
Young LF, Martin KR (2006) Time-dependent resveratrol-mediated mRNA and protein expression associated with cell cycle in WR-21 cells containing mutated human c-Ha-Ras. Mol Nutr Food Res 50:70–77
Traversi G, Fiore M, Percario Z et al (2017) The resveratrol analogue trimethoxystilbene inhibits cancer cell growth by inducing multipolar cell mitosis. Mol Carcinog 56:1117–1126
Farhood B, Goradel NH, Mortezaee K et al (2019) Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal 13:3–16
Giménez-Bastida JA, Ávila-Gálvez MÁ, Espín JC et al (2019) Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol Nutr Food Res 63:1900629
Chen K-Y, Chen C-C, Chang Y-C et al (2019) Resveratrol induced premature senescence and inhibited epithelial-mesenchymal transition of cancer cells via induction of tumor suppressor Rad9. PLoS ONE 14:e0219317
Ji S, Zheng Z, Liu S et al (2018) Resveratrol promotes oxidative stress to drive DLC1 mediated cellular senescence in cancer cells. Exp Cell Res 370:292–302
Li B, Hou D, Guo H et al (2017) Resveratrol sequentially induces replication and oxidative stresses to drive p53-CXCR2 mediated cellular senescence in cancer cells. Sci Rep 7:208
Fang Y, Demarco VG, Nicholl MB (2012) Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci 103:1090–1098
Sayd S, Thirant C, El-Habr EA et al (2014) Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev Rep 10:103–113
Alobaedi OH, Talib WH, Basheti IA (2017) Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac J Trop Med 10:400–408
San Hipólito-Luengo Á, Alcaide A, Ramos-González M et al (2017) Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutr Cancer 69:1019–1027
Scifo C, Cardile V, Russo A et al (2004) Resveratrol and propolis as necrosis or apoptosis inducers in human prostate carcinoma cells. Oncol Res 14:415–426
Scifo C, Milasi A, Guarnera A et al (2005) Resveratrol and propolis extract: an insight into the morphological and molecular changes induced in DU145 cells. Oncol Res 15:409–421
Acknowledgements
Supported by Cooperative Foundation of Shaoyang University.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparing first draft. The scientific edition performed by MN. All authors wrote and approved the article.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human and/or animal participants
This article does not contain human or animal studies performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Li, M., Tang, C. et al. Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis 26, 561–573 (2021). https://doi.org/10.1007/s10495-021-01689-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01689-7