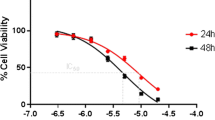
Abstract
Breast cancer accounts for 1.4 million new cases every year. Triple-negative breast cancer (TNBC) is one the leading cause of mortality in developing countries and is associated with early age onset (under 40 years old). Chemotherapy has a poor success rate in patients with TNBC as compared to other types of breast cancers. It is due to the lack of expression of three validated molecular markers for breast cancer, the estrogen and progesterone receptors, and the amplification of HER-2/Neu. Therefore, a clear need exists for a greater understanding of TNBC at all levels and for the development of better therapies. We have studied the anti-tumor effects of a potential drug, maslinic acid, which can be extracted from olive oil industry waste. This natural product showed inhibitory effect at concentrations ranging from 30 to 50 µM within 24 h. It exhibited divergent effects in cell cycle progression for the MCF7 (estrogen positive) cell line when compared with TNBCs like MDA-MB-231 and MDA-MB-468. Also, maslinic acid treatment altered the mitochondrial membrane electrochemical potential and the reactive oxygen species (ROS) levels to cause a caspase-independent programmed cell death. In silico approaches and immunoblotting suggested the involvement of the MAPK pathway explaining the variability in cell cycle progression along with the apoptotic cell death caused by maslinic acid.







Similar content being viewed by others
References
Boyle P (2012) Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 23:vi7-vi12. https://doi.org/10.1093/annonc/mds187
Marra A, Viale G, Curigliano G (2019) Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med 17(1):90. https://doi.org/10.1186/s12916-019-1326-5
Yao H, He G, Yan S, Chen C, Song L, Rosol TJ, Deng X (2017) Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget 8(1):1913–1924. https://doi.org/10.18632/oncotarget.12284
Wahba HA, El-Hadaad HA (2015) Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med 12(2):106–116. https://doi.org/10.7497/j.issn.2095-3941.2015.0030
Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C (2003) Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomark Prevent 12(10):1091–1094
Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D (2000) Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomark Prevent 9(9):869–873
Owen R, Haubner R, Wuertele G, Hull E, Spiegelhalder B, Bartsch H (2004) Olives and olive oil in cancer prevention. Eur J Cancer Prevent 13:319–326. https://doi.org/10.1097/01.cej.0000130221.19480.7e
Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, Weiderpass E, Adami HO (2006) Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr 96(2):384–392
Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A (2007) Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP diet and health study. Arch Intern Med 167(22):2461–2468. https://doi.org/10.1001/archinte.167.22.2461
Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, Trichopoulos D, Ec G (2008) Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. Br J Cancer 99(1):191–195. https://doi.org/10.1038/sj.bjc.6604418
Keys A, Menotti A, Aravanis C, Blackburn H, Djordevic BS, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Kimura N et al (1984) The seven countries study: 2,289 deaths in 15 years. Prevent Med 13(2):141–154
Reyes FJ, Centelles JJ, Lupianez JA, Cascante M (2006) (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett 580(27):6302–6310. https://doi.org/10.1016/j.febslet.2006.10.038
Nieto FR, Cobos EJ, Entrena JM, Parra A, Garcia-Granados A, Baeyens JM (2013) Antiallodynic and analgesic effects of maslinic acid, a pentacyclic triterpenoid from Olea europaea. J Nat Prod 76(4):737–740. https://doi.org/10.1021/np300783a
Marquez Martin A, de la Puerta VR, Fernandez-Arche A, Ruiz-Gutierrez V (2006) Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic Res 40(3):295–302. https://doi.org/10.1080/10715760500467935
Montilla MP, Agil A, Navarro MC, Jiménez MI, García-Granados A, Parra A, Cabo MM (2003) Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med 69(5):472–474. https://doi.org/10.1055/s-2003-39698
Xu HX, Zeng FQ, Wan M, Sim KY (1996) Anti-HIV triterpene acids from Geum japonicum. J Nat Prod 59(7):643–645. https://doi.org/10.1021/np960165e
Rodriguez-Rodriguez R, Perona JS, Herrera MD, Ruiz-Gutierrez V (2006) Triterpenic compounds from “Orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J Agric Food Chem 54(6):2096–2102. https://doi.org/10.1021/jf0528512
Juan ME, Planas JM, Ruiz-Gutierrez V, Daniel H, Wenzel U (2008) Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br J Nutr 100(1):36–43. https://doi.org/10.1017/s0007114508882979
Reyes-Zurita FJ, Rufino-Palomares EE, Lupianez JA, Cascante M (2009) Maslinic acid, a natural triterpene from Olea europaea L., Induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett 273(1):44–54. https://doi.org/10.1016/j.canlet.2008.07.033
Reyes-Zurita FJ, Pachon-Pena G, Lizarraga D, Rufino-Palomares EE, Cascante M, Lupianez JA (2011) The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 11:154. https://doi.org/10.1186/1471-2407-11-154
Allouche Y, Warleta F, Campos M, Sanchez-Quesada C, Uceda M, Beltran G, Gaforio JJ (2011) Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J Agric Food Chem 59(1):121–130. https://doi.org/10.1021/jf102319y
Wu DM, Zhao D, Li DZ, Xu DY, Chu WF, Wang XF (2011) Maslinic acid induces apoptosis in salivary gland adenoid cystic carcinoma cells by Ca2+-evoked p38 signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol 383(3):321–330. https://doi.org/10.1007/s00210-011-0598-x
Li C, Yang Z, Zhai C, Qiu W, Li D, Yi Z, Wang L, Tang J, Qian M, Luo J, Liu M (2010) Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor alpha by inhibiting NF-kappaB signaling pathway. Mol Cancer 9:73. https://doi.org/10.1186/1476-4598-9-73
Parra A, Rivas F, Martin-Fonseca S, Garcia-Granados A, Martinez A (2011) Maslinic acid derivatives induce significant apoptosis in b16f10 murine melanoma cells. Eur J Med Chem 46(12):5991–6001. https://doi.org/10.1016/j.ejmech.2011.10.011
Hsum YW, Yew WT, Hong PL, Soo KK, Hoon LS, Chieng YC, Mooi LY (2011) Cancer chemopreventive activity of maslinic acid: suppression of COX-2 expression and inhibition of NF-kappaB and AP-1 activation in Raji cells. Planta Med 77(2):152–157. https://doi.org/10.1055/s-0030-1250203
Park SY, Nho CW, Kwon DY, Kang Y-H, Lee KW, Park JHY (2012) Maslinic acid inhibits the metastatic capacity of DU145 human prostate cancer cells: possible mediation via hypoxia-inducible factor-1α signalling. Br J Nutr 109(2):210–222. https://doi.org/10.1017/S0007114512000967
Mooi LY, Yew WT, Hsum YW, Soo KK, Hoon LS, Chieng YC (2012) Suppressive effect of maslinic acid on PMA-induced protein kinase C in human B-lymphoblastoid cells. Asian Pac J Cancer Prevent 13(4):1177–1182. https://doi.org/10.7314/apjcp.2012.13.4.1177
Twentyman PR, Luscombe M (1987) A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 56(3):279–285. https://doi.org/10.1038/bjc.1987.190
Liu X, Ouyang S, Yu B, Liu Y, Huang K, Gong J, Zheng S, Li Z, Li H, Jiang H (2010) PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucl Acids Res 38:W609–614. https://doi.org/10.1093/nar/gkq300
Chen Z, Wang X, Zhu W, Cao X, Tong L, Li H, Xie H, Xu Y, Tan S, Kuang D, Ding J, Qian X (2011) Acenaphtho[1,2-b]pyrrole-based selective fibroblast growth factor receptors 1 (FGFR1) inhibitors: design, synthesis, and biological activity. J Med Chem 54(11):3732–3745. https://doi.org/10.1021/jm200258t
Georgieva M, Zlatkov B, Zlatkov A (2014) Applying pharmmapper server as tool for drug target identification for some diphenylmethylpiperazine amides. World J Pharm Pharma Sci 3:94
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucl Acids Res 28(1):235–242. https://doi.org/10.1093/nar/28.1.235
Gupta A, Jain R, Wahi D, Goyal S, Jamal S, Grover A (2015) Abrogation of AuroraA-TPX2 by novel natural inhibitors: molecular dynamics-based mechanistic analysis. J Recept Signal Transduct Res 35(6):626–633. https://doi.org/10.3109/10799893.2015.1041645
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput-Aided Mol Des 27(3):221–234. https://doi.org/10.1007/s10822-013-9644-8
Elokely KM, Doerksen RJ (2013) Docking challenge: protein sampling and molecular docking performance. J Chem Inf Model 53(8):1934–1945. https://doi.org/10.1021/ci400040d
Halgren TA (2009) Identifying and characterizing binding sites and assessing druggability. J Chem Inf Model 49(2):377–389. https://doi.org/10.1021/ci800324m
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749. https://doi.org/10.1021/jm0306430
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring 2 Enrichment factors in database screening. J Med Chem 47(7):1750–1759. https://doi.org/10.1021/jm030644s
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49(21):6177–6196. https://doi.org/10.1021/jm051256o
Lua RC, Lichtarge O (2010) PyETV: a PyMOL evolutionary trace viewer to analyze functional site predictions in protein complexes. Bioinformatics 26(23):2981–2982. https://doi.org/10.1093/bioinformatics/btq566
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8(2):127–134
Hetenyi C, Szabo Z, Klement E, Datki Z, Kortvelyesi T, Zarandi M, Penke B (2002) Pentapeptide amides interfere with the aggregation of beta-amyloid peptide of Alzheimer's disease. Biochem Biophys Res Commun 292(4):931–936. https://doi.org/10.1006/bbrc.2002.6745
Cavasotto CN (2012) Normal mode-based approaches in receptor ensemble docking. Methods Mol Biol (Clifton, NJ) 819:157–168. https://doi.org/10.1007/978-1-61779-465-0_11
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19(14):1639–1662. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639:AID-JCC10>3.0.CO;2-B
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Abramoff M, Magalhães P, Ram SJ (2003) Image processing with imageJ biophoton Int 11:36–42
Chambard J-C, Lefloch R, Pouysségur J, Lenormand P (2007) ERK implication in cell cycle regulation. Biochim Biophys Acta (BBA) 1773(8):1299–1310. https://doi.org/10.1016/j.bbamcr.2006.11.010
Yamamoto T, Ebisuya M, Ashida F, Okamoto K, Yonehara S, Nishida E (2006) Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr Biol 16(12):1171–1182. https://doi.org/10.1016/j.cub.2006.04.044
Jones SM, Kazlauskas A (2001) Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol 3(2):165–172. https://doi.org/10.1038/35055073
Keenan SM, Bellone C, Baldassare JJ (2001) Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase. J Biol Chem 276(25):22404–22409. https://doi.org/10.1074/jbc.M100409200
Lents NH, Keenan SM, Bellone C, Baldassare JJ (2002) Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J Biol Chem 277(49):47469–47475. https://doi.org/10.1074/jbc.M207425200
Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB (2012) Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 16(11):1295–1322. https://doi.org/10.1089/ars.2011.4414
Cheung EC, Slack RS (2004) Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE 2004(251):PE45. https://doi.org/10.1126/stke.2512004pe45
Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG (2005) Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J Biol Chem 280(36):31498–31507. https://doi.org/10.1074/jbc.M505537200
Yue W, Wang J-P, Conaway M, Masamura S, Li Y, Santen RJ (2002) Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology 143(9):3221–3229. https://doi.org/10.1210/en.2002-220186
Kumari R, Chouhan S, Singh S, Chhipa RR, Ajay AK, Bhat MK (2017) Constitutively activated ERK sensitizes cancer cells to doxorubicin: involvement of p53-EGFR-ERK pathway. J Biosci 42(1):31–41
Boldt S, Weidle UH, Kolch W (2002) The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis 23(11):1831–1838. https://doi.org/10.1093/carcin/23.11.1831
Hollmann CA, Owens T, Nalbantoglu J, Hudson TJ, Sladek R (2006) Constitutive activation of extracellular signal-regulated kinase predisposes diffuse large B-cell lymphoma cell lines to CD40-mediated cell death. Cancer Res 66(7):3550–3557. https://doi.org/10.1158/0008-5472.can-05-2498
Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ (2008) Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 75(10):1946–1958. https://doi.org/10.1016/j.bcp.2008.02.016
Acknowledgements
RJ is thankful to Department of Health Research, Ministry of Health & Family Welfare, India for Young Scientist position and the financial support for conducting research. AG is grateful to University Grants Commission, India for the Faculty Recharge Position. RJ & AG is thankful to Jawaharlal Nehru University for usage of computational facility. Authors are also thankful to Dr. Sukriti Goyal for the help in Autodock studies.
Author information
Authors and Affiliations
Contributions
RJ & AG conceived and designed the study. RJ carried out the experiments and analysed the results. RJ wrote the manuscript. RJ and AG reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, R., Grover, A. Maslinic acid differentially exploits the MAPK pathway in estrogen-positive and triple-negative breast cancer to induce mitochondrion-mediated, caspase-independent apoptosis. Apoptosis 25, 817–834 (2020). https://doi.org/10.1007/s10495-020-01636-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01636-y