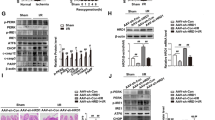
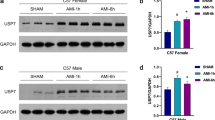
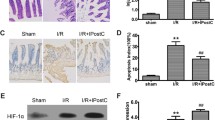
Abstract
Epithelial apoptosis is an important factor in intestinal ischemia–reperfusion (I/R) injury. Heat shock factor 1 (HSF1) is a classical stress response factor that directly regulates the transcription of heat shock proteins (HSPs) under stress conditions. Although HSPs are involved in protecting the intestine against I/R, the mechanism whereby HSF1 is regulated in I/R is poorly understood. Here, we show that the ubiquitin ligase FBXW7 targets HSF1 for ubiquitination and degradation in intestinal I/R. In this study, we found that FBXW7 expression was upregulated at the transcriptional level in intestinal mucosae subjected to I/R. In Caco-2 and IEC-6 cells subjected to hypoxia/reoxygenation (H/R), a high FBXW7 level led to excessive HSF1 ubiquitination and degradation. FBXW7 knockdown attenuated HSF1 ubiquitination and downregulation and accelerated HSPB1 and HSP70 expression. In addition, FBXW7 deletion alleviated the apoptosis of intestinal epithelial cells, as evidenced by decreased activation of caspase-3 and caspase-9. The results suggest that FBXW7 suppression protects against intestinal I/R, at least partly through the HSF1/HSP pathway. These findings indicate that FBXW7 may be a potential therapeutic target for inhibiting intestinal mucosa apoptosis during intestinal I/R.





Similar content being viewed by others
References
Reino DC, Palange D, Feketeova E et al (2012) Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock 38:107–114. https://doi.org/10.1097/SHK.0b013e318257123a
Tadros T, Traber DL, Heggers JP, Herndon DN (2003) Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg 237: 101–109. https://doi.org/10.1097/01.SLA.0000041039.16815.69
Gibot S, Massin F, Alauzet C, Montemont C, Lozniewski A, Bollaert PE, Levy B (2008) Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit Care Med 36:504–510. https://doi.org/10.1097/01.CCM.0B013E318161FAF3
Jin Y, Blikslager AT (2015) ClC-2 regulation of intestinal barrier function: translation of basic science to therapeutic target. Tissue Barriers 3:e1105906. https://doi.org/10.1080/21688370.2015.1105906
Azuara D, Sola A, Hotter G, Calatayud L, de Oca J (2005) Apoptosis inhibition plays a greater role than necrosis inhibition in decreasing bacterial translocation in experimental intestinal transplantation. Surgery 137:85–91. https://doi.org/10.1016/j.surg.2004.06.008
Micel LN, Tentler JJ, Smith PG, Eckhardt GS (2013) Role of ubiquitin ligases and the proteasome in oncogenesis: novel targets for anticancer therapies. J Clin Oncol 31:1231–1238. https://doi.org/10.1200/JCO.2012.44.0958
Schwartz AL, Ciechanover A (2009) Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol 49:73–96. https://doi.org/10.1146/annurev.pharmtox.051208.165340
Weissman AM, Shabek N, Ciechanover A (2011) The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat Rev Mol Cell Biol 12:605–620. https://doi.org/10.1038/nrm3173
Lipkowitz S, Weissman AM (2011) RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer 11:629–643. https://doi.org/10.1038/nrc3120
Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6:9–20. https://doi.org/10.1038/nrm1547
Hotton SK, Callis J (2008) Regulation of cullin RING ligases. Annu Rev Plant Biol 59:467–489. https://doi.org/10.1146/annurev.arplant.58.032806.104011
Bosu DR, Kipreos ET (2008) Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div 3:7. https://doi.org/10.1186/1747-1028-3-7
Heo J, Eki R, Abbas T (2016) Deregulation of F-box proteins and its consequence on cancer development, progression and metastasis. Semin Cancer Biol 36:33–51. https://doi.org/10.1016/j.semcancer.2015.09.015
Welcker M, Clurman BE (2008) FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8:83–93. https://doi.org/10.1038/nrc2290
Minella AC, Clurman BE (2005) Mechanisms of tumor suppression by the SCF(Fbw7). Cell Cycle 4:1356–1359. https://doi.org/10.4161/cc.4.10.2058
Lau AW, Fukushima H, Wei W (2012) The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci (Landmark Ed) 17:2197–2212
Diefenbacher ME, Chakraborty A, Blake SM, Mitter R, Popov N, Eilers M, Behrens A (2015) Usp28 counteracts Fbw7 in intestinal homeostasis and cancer. Cancer Res 75:1181–1186. https://doi.org/10.1158/0008-5472.CAN-14-1726
Olson BL, Hock MB, Ekholm-Reed S, Wohlschlegel JA, Dev KK, Kralli A, Reed SI (2008) SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis. Genes Dev 22:252–264. https://doi.org/10.1101/gad.1624208
Ekholm-Reed S, Goldberg MS, Schlossmacher MG, Reed SI (2013) Parkin-dependent degradation of the F-box protein Fbw7beta promotes neuronal survival in response to oxidative stress by stabilizing Mcl-1. Mol Cell Biol 33:3627–3643. https://doi.org/10.1128/MCB.00535-13
Kannan MB, Dodard-Friedman I, Blank V (2015) Stringent control of NFE2L3 (nuclear factor, erythroid 2-like 3; NRF3) protein degradation by FBW7 (F-box/WD repeat-containing protein 7) and glycogen synthase kinase 3 (GSK3). J Biol Chem 290:26292–26302. https://doi.org/10.1074/jbc.M115.666446
Cassavaugh JM, Hale SA, Wellman TL, Howe AK, Wong C, Lounsbury KM (2011) Negative regulation of HIF-1alpha by an FBW7-mediated degradation pathway during hypoxia. J Cell Biochem 112:3882–3890. https://doi.org/10.1002/jcb.23321
Pelorosso FG, Balmain A (2010) C/EBPdelta: friend or foe? a novel role for C/EBPdelta in metastasis. EMBO J 29:4063–4065. https://doi.org/10.1038/emboj.2010.308
Vihervaara A, Sistonen L (2014) HSF1 at a glance. J Cell Sci 127:261–266. https://doi.org/10.1242/jcs.132605
Neudegger T, Verghese J, Hayer-Hartl M, Hartl FU, Bracher A (2016) Structure of human heat-shock transcription factor 1 in complex with DNA. Nat Struct Mol Biol 23:140–146. https://doi.org/10.1038/nsmb.3149
Anckar J, Sistonen L (2011) Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80:1089–1115. https://doi.org/10.1146/annurev-biochem-060809-095203
Sandqvist A, Bjork JK, Akerfelt M et al (2009) Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol Biol Cell 20:1340–1347
Ao L, Zhai Y, Jin C, Cleveland JC, Fullerton DA, Meng X (2016) Attenuated recovery of contractile function in aging hearts following global ischemia/reperfusion: role of extracellular HSP27 and TLR4. Mol Med. https://doi.org/10.2119/molmed.2016.00204
O’Neill S, Hughes J (2014) Heat-shock protein-70 and regulatory T cell-mediated protection from ischemic injury. Kidney Int 85:5–7. https://doi.org/10.1038/ki.2013.304
Oksala NK, Kaarniranta K, Tenhunen JJ, Tiihonen R, Heino A, Sistonen L, Paimela H, Alhava E (2002) Reperfusion but not acute ischemia in pig small intestine induces transcriptionally mediated heat shock response in situ. Eur Surg Res 34:397–404. https://doi.org/10.1159/000065708
Kourtis N, Moubarak RS, Aranda-Orgilles B et al (2015) FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol 17:322–332. https://doi.org/10.1038/ncb3121
Gomez-Pastor R, Burchfiel ET, Neef DW et al (2017) Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat Commun 8:14405. https://doi.org/10.1038/ncomms14405
Skaar JR, Pagan JK, Pagano M (2013) Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol 14:369–381. https://doi.org/10.1038/nrm3582
Cuzzocrea S, Mazzon E, Esposito E, Muia C, Abdelrahman M, Di Paola R, Crisafulli C, Bramanti P, Thiemermann C (2007) Glycogen synthase kinase-3 beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med 33:880–893. https://doi.org/10.1007/s00134-007-0595-1
Ban K, Peng Z, Kozar RA (2013) Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS ONE 8:e76790. https://doi.org/10.1371/journal.pone.0076790
Wang G, Yao J, Li Z et al (2016) miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species Accumulation and apoptosisvia activation of SIRT1 signaling. Antioxid Redox Sign 24:961–973. https://doi.org/10.1089/ars.2015.6492
Bruey JM, Ducasse C, Bonniaud P et al (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652. https://doi.org/10.1038/35023595
Xanthoudakis S, Nicholson DW (2000) Heat-shock proteins as death determinants. Nat Cell Biol 2:E163–E165. https://doi.org/10.1038/35023643
Lane JS, Todd KE, Lewis MP, Gloor B, Ashley SW, Reber HA, McFadden DW, Chandler CF (1997) Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 122:288–294
Schwartz AL, Ciechanover A et al (2009) Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol. https://doi.org/10.1146/annurev.pharmtox.051208.165340
Rafiq K, Kolpakov MA, Seqqat R et al (2014) c-Cbl inhibition improves cardiac function and survival in response to myocardial ischemia. Circulation 129:2031–2043. https://doi.org/10.1161/CIRCULATIONAHA.113.007004
Dong W, Wang H, Shahzad K et al (2015) Activated protein C ameliorates renal ischemia-reperfusion injury by restricting Y-box binding protein-1 ubiquitination. J Am Soc Nephrol 26:2789–2799. https://doi.org/10.1681/ASN.2014080846
Zhi-Yong S, Dong YL, Wang XH (1992) Bacterial translocation and multiple system organ failure in bowel ischemia and reperfusion. J Trauma 32:148–153
Wang L, Ye X, Liu Y, Wei W, Wang Z (2014) Aberrant regulation of FBW7 in cancer. Oncotarget 5:2000–2015. https://doi.org/10.18632/oncotarget.1859
Zhao X, Hirota T, Han X et al (2016) Circadian amplitude regulation via FBXW7-targeted REV-ERBalpha degradation. CELL 165:1644–1657. https://doi.org/10.1016/j.cell.2016.05.012
Gray CC, Amrani M, Yacoub MH (1999) Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int J Biochem Cell Biol 31:559–573
Latchman DS (2001) Heat shock proteins and cardiac protection. Cardiovasc Res 51:637–646
Pockley AG (2002) Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105:1012–1017
Jeong YJ, Jung MG, Son Y, Jang JH, Lee YJ, Kim SH, Ko YG, Lee YS, Lee HJ (2015) Coniferyl aldehyde attenuates radiation enteropathy by inhibiting cell death and promoting endothelial cell function. PLoS ONE 10:e128552. https://doi.org/10.1371/journal.pone.0128552
Akagi R, Ohno M, Matsubara K, Fujimoto M, Nakai A, Inouye S (2013) Glutamine protects intestinal barrier function of colon epithelial cells from ethanol by modulating Hsp70 expression. Pharmacology 91:104–111. https://doi.org/10.1159/000345930
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81671954 and 81372037 to Xiaofeng Tian and No. 81501699 to Feng Zhang).
Author information
Authors and Affiliations
Contributions
XT, WT and JY designed the study. WT, HZ, LL and WZ performed the experiments. WT, FZ, YL, DF, ZL and JY analyzed and interpreted the data. XT, JY and FZ wrote and revised the manuscript. XT provided financial support. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tan, W., Zhao, H., Zhang, F. et al. Inhibition of the ubiquitination of HSF1 by FBXW7 protects the intestine against ischemia–reperfusion injury. Apoptosis 23, 667–678 (2018). https://doi.org/10.1007/s10495-018-1484-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1484-5