Abstract
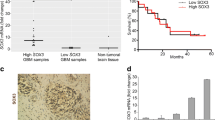
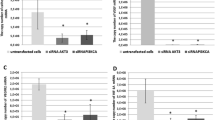
Strong 14-3-3 zeta protein expression plays an important role in tumorigenesis, including in the maintenance of cell growth, resistance increase, and the prevention of apoptosis. In this study, we focus on two targets: (1) the expression of 14-3-3 zeta in the different grades of human astrocytoma (II–IV), (2) suppression of 14-3-3 zeta protein expression in glioblastoma derived astrocytes by 14-3-3 zeta shRNA lentiviral particles. The tissues of human astrocytoma were provided from 30 patients (ten of each grade of astrocytoma). Control tissues were obtained from the peritumoral brain zone of those patients with glioblastoma. The protein and mRNA expression levels of each astrocytoma grade were assessed via western blotting and RT-PCR, respectively. Results indicated that 14-3-3 zeta was significantly expressed in glioblastoma multiforme (grade IV) and 14-3-3 zeta expression levels enhanced according to the increase of astrocytoma malignancy. In the cellular study for knock down of the 14-3-3 zeta protein, surgical biopsy of glioblastoma was used to isolate primary astrocyte. Astrocytes were transduced with 14-3-3 zeta shRNA or non-targeted shRNA lentiviral particles. Furthermore, reduction of the 14-3-3 zeta protein expression in the astrocytes evaluated through qRT-PCR and western blot after transduction of 14-3-3 zeta shRNA lentiviral particles. Moreover, apoptosis properties, including DNA fragmentation and ratio increase of Bax/Bcl-2 were observed in astrocytes following reduction of 14-3-3 zeta protein expression. Further observation indicated that the mitochondrial pathway through release of cytochorome c and caspase-3 activity was involved in the apoptosis induction. Hence, this study demonstrates a key role of the 14-3-3 zeta protein in tumorigenesis but also indicates that 14-3-3 zeta can be considered as a target for the astrocytoma treatment specially glioblastoma.








Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G, Deimling AV, Branger DF, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Cao WD, Zhang X, Zhang JN, Yang ZJ, Zhen HN, Cheng G et al (2006) Immunocytochemical detection of 14-3-3 in primary nervous system tumors. J Neuro-Oncol 77:125–130
Van Hemert MJ, Steensma HY, van Heusden GP (2001) 14-3-3 proteins: key regulators of cell division, signaling and apoptosis. Bioessays 23:936–946
Tzivion G, Avruch J (2002) 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem 277:3061–3064
Van Heusden GP (2005) 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life 57:623–629
Nishimura Y, Komatsu S, Ichikawa D, Nagata H, Hirajima S, Takeshita H, Kawaguchi T, Arita T et al (2013) Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer 108:1324–1331
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H et al (2009) 14-3-3 zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 69:3425–3432
Matta A, Masui O, Siu KW, Ralhan R (2016) Identification of 14-3-3 zeta associated protein networks in oral cancer. Proteomics 16:1079–1089
Cao L, Cao W, Zhang W, Lin H, Yang X, Zhen H, Cheng J, Dong W et al (2008) Identification of 14-3-3 protein isoforms in human astrocytoma by immunohistochemistry. Neurosci Lett 432:94–99
Rapp UR, Fischer A, Rennefahrt UE, Hekman M, Albert S (2007) BAD association with membranes is regulated by Raf kinases and association with 14-3-3 proteins. Adv Enzyme Regul 47:281–285
Wolvetang EJ, Pera MF, Zuckerman KS (2007) Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun 363:610–615
Xu Y, Linde A, Larsson O, Thormeyer D, Elmen J, Wahlestedt C, Liang Z (2004) Functional comparison of single- and double-stranded siRNAs in mammalian cells. Biochem Biophys Res Commun 316:680–687
Lemée JM, Com E, Clavreul A, Avril T, Quillien V, de Tayrac M et al (2013) Proteomic analysis of glioblastomas: what is the best brain control sample? J Proteom 24:165–173
Hashemi M, Hadjighassem MR (2017) Preparation of primary astrocyte culture derived from human glioblastoma multiforme specimen. Bio-protocol 7:1–9
Hashemi M, Fallah A, Aghayan HR, Arjmand B, Yazdani N, Verdi j, Ghodsi SM, Miri SM, Hadjighassem M (2016) A new approach in gene therapy of glioblastoma multiforme: human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Mol Neurobiol 53:5118–5128
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat Protoc 2:287–295
Cao W, Yang X, Zhou J, Teng Z, Cao L, Zhang X, Fei Z (2010) Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis 15:230–241
Yang X, Cao W, Lin H, Zhang W, Lin W, Cao L et al (2009) Isoform-specific expression of 14-3-3 proteins in human astrocytoma. J Neurol Sci 276:54–59
Fulda S (2009) Apoptosis pathways and neuroblastoma therapy. Curr Pharm Des 15:430–435
Fulda S (2009) Tumor resistance to apoptosis. Int J Cancer 124:511–515
Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y (2003) 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278:2058–2065
Lee YK, Hur W, Lee SW, Hong SW, Kim SW, Choi JE (2014) Knockdown of 14-3-3ζ enhances radiosensitivity and radio-induced apoptosis in CD133+ liver cancer stem cells. Exp Mol Med 46:e77
Hannus M, Beitzinger M, Engelmann J, Weickert M, Spang R, Hannus S et al (2014) siPools: highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res 42:8049–8061
Funding
This study was funded by functional neurosurgery research center, Shahid Beheshti University of medical sciences (Grant Number: 11974).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in study according to the Ethical Commission of the Shahid Beheshti University of Medical Sciences. Ethical code of study is IR.SBMU.RETECH.REC.1396.744.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hashemi, M., Zali, A., Hashemi, J. et al. Down-regulation of 14-3-3 zeta sensitizes human glioblastoma cells to apoptosis induction. Apoptosis 23, 616–625 (2018). https://doi.org/10.1007/s10495-018-1476-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1476-5