Abstract
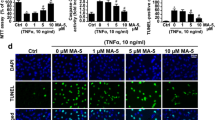
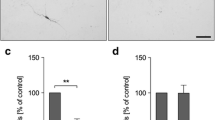
It has been reported that activation of NF-κB is involved in excitotoxicity; however, it is not fully understood how NF-κB contributes to excitotoxicity. The aim of this study is to investigate if NF-κB contributes to quinolinic acid (QA)-mediated excitotoxicity through activation of microglia. In the cultured primary cortical neurons and microglia BV-2 cells, the effects of QA on cell survival, NF-κB expression and cytokines production were investigated. The effects of BV-2-conditioned medium (BCM) on primary cortical neurons were examined. The effects of pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB, and minocycline (MC), an inhibitor of microglia activation, on QA-induced excitotoxicity were assessed. QA-induced NF-κB activation and TNF-α secretion, and the roles of TNF-α in excitotoxicity were studied. QA at the concentration below 1 mM had no apparent toxic effects on cultured primary neurons or BV-2 cells. However, addition of QA-primed BCM to primary neurons did aggravate QA-induced excitotoxicity. The exacerbation of QA-induced excitotoxicity by BCM was partially ameliorated by inhibiting NF-κB and microglia activation. QA induced activation of NF-κB and upregulation of TNF-α in BV-2 cells. Addition of recombinant TNF-α mimicked QA-induced excitotoxic effects on neurons, and neutralizing TNF-α with specific antibodies partially abolished exacerbation of QA-induced excitotoxicity by BCM. These studies suggested that QA activated microglia and upregulated TNF-α through NF-κB pathway in microglia. The microglia-mediated inflammatory pathway contributed, at least in part, to QA-induced excitotoxicity.






Similar content being viewed by others
References
Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164:719–721
Posada-Duque RA, Barreto GE, Cardona-Gomez GP (2014) Protection after stroke: cellular effectors of neurovascular unit integrity. Front Cell Neurosci 8:231
Sámano C, Kaur J, Nistri A (2016) A study of methylprednisolone neuroprotection against acute injury to the rat spinal cord in vitro. Neuroscience 315:136–149
Beste C, Stock AK, Ness V, Hoffmann R, Saft C (2015) Evidence for divergent effects of neurodegeneration in Huntington’s disease on attentional selection and neural plasticity: implications for excitotoxicity. Brain Struct Funct 220(3):1437–1447
Litim N, Morissette M, Di Paolo T (2016) Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease: an update from the last 5 years of research. Neuropharmacology S0028–3908(16): 30108–30113
Luchtman D, Gollan R, Ellwardt E, Birkenstock J, Robohm K, Siffrin V, Zipp F (2016) In vivo and in vitro effects of multiple sclerosis immunomodulatory therapeutics on glutamatergicexcitotoxicity. J Neurochem 136(5):971–980
Pallo SP, DiMaio J, Cook A, Nilsson B, Johnson GV (2016) Mechanisms of tau and Aβ-induced excitotoxicity. Brain Res 1634:119–131
Zhang W, Zhang L, Liang B, Schroeder D, Zhang ZW, Cox GA, Li Y, Lin DT (2016) Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci 19(4):557–559
Feng Q, Ma Y, Mu S, Wu J, Chen S, Ouyang L, Lei W (2014) Specific reactions of different striatal neuron types in morphology induced by quinolinic acid in rats. PLoS ONE 9:e91512
Kubicova L, Hadacek F, Weckwerth W, Chobot V (2015) Effects of endogenous neurotoxin quinolinic acid on reactive oxygen species production by Fenton reaction catalyzed by iron or copper. J Organomet Chem 782:111–115
Schwarcz R, Whetsell WO Jr, Mangano RM (1983) Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219:316–318
Sundaram G, Brew BJ, Jones SP, Adams S, Lim CK, Guillemin GJ (2014) Quinolinic acid toxicity on oligodendroglial cells: relevance for multiple sclerosis and therapeutic strategies. J Neuroinflammation 11:204
Beal MF, Ferrante RJ, Swartz KJ, Kowall NW (1991) Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. J Neurosci 11:1649–1659
Scattoni ML, Valanzano A, Popoli P, Pezzola A, Reggio R, Calamandrei G (2004) Progressive behavioural changes in the spatial open-field in the quinolinic acid rat model of Huntington’s disease. Behav Brain Res 152:375–383
Parrott JM, O’Connor JC (2015) Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry 6:116
Sathyasaikumar KV, Stachowski EK, Amori L, Guidetti P, Muchowski PJ, Schwarcz R (2010) Dysfunctional kynurenine pathway metabolism in the R6/2 mouse model of Huntington’s disease. J Neurochem 113:1416–1425
González Esquivel D, Ramírez-Ortega D, Pineda B, Castro N, Ríos C, Pérez de la Cruz V (2017) Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 112(Pt B):331–345
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30:379–387
Dong XX, Wang YR, Qin S, Liang ZQ, Liu BH, Qin ZH, Wang Y (2012) p53 Mediates autophagy activation and mitochondria dysfunction in kainic acid-induced excitotoxicity in primary striatal neurons. Neuroscience 207:52–64
Wang Y, Dong XX, Cao Y, Liang ZQ, Han R, Wu JC, Gu ZL, Qin ZH (2009) P53 induction contributes to excitotoxic neuronal death in rat striatum through apoptotic and autophagic mechanisms. Eur J Neurosci 30:2258–2270
Wang Y, Han R, Liang ZQ, Wu JC, Zhang XD, Gu ZL, Qin ZH (2008) An autophagic mechanism is involved in apoptotic death of rat striatal neurons induced by the non-N-methyl-D-aspartate receptor agonist kainic acid. Autophagy 4:214–226
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15:1382–1402
Wang Y, Qin ZH (2013) Coordination of autophagy with other cellular activities. Acta Pharmacol Sin 34:585–594
Wang YR, Qin S, Liang ZQ, Han R, Wu JC, Qin ZH, Wang Y (2013) Cathepsin L plays a role in quinolinic acid-induced NF-κB activation and excitotoxicity in rats striatal neurons. PLoS ONE 8:e75702
Nakai M, Qin ZH, Wang YM, Chase TN (2000) NMDA and non-NMDA receptor-stimulated I kappa B-alpha degradation: differential effects of the caspase-3 inhibitor DEVD center dot CHO, ethanol and free radical scavenger OPC-14117. Brain Res 859:207–216
Nakai M, Qin ZH, Wang Y, Chase TN (1999) Free radical scavenger OPC-14117 attenuates quinolinic acid-induced NF-kappaB activation and apoptosis in rat striatum. Brain Res Mol Brain Res 64:59–68
Wang Y, Gu ZL, Cao Y, Liang ZQ, Han R et al (2006) Lysosomal enzyme cathepsin B is involved in kainic acid-induced excitotoxicity in rat striatum. Brain Res 1071:245–249
Wood LB, Winslow AR, Proctor EA, McGuone D, Mordes DA, Frosch MP, Hyman BT, Lauffenburger DA, Haigis KM (2015) Identification of neurotoxic cytokines by profiling Alzheimer’s disease tissues and neuron culture viability screening. Sci Rep 5:16622
Albensi BC, Mattson MP (2000) Evidence for the involvement of TNF and NFkappaB in hippocampal synaptic plasticity. Synapse 35:151–159
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4:702–710
Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M (2011) Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci 33:1483–1492
Dumont AO, Goursaud S, Desmet N, Hermans E (2014) Differential regulation of glutamate transporter subtypes by pro-inflammatory cytokine TNF-alpha in cortical astrocytes from a rat model of amyotrophic lateral sclerosis. PLoS ONE 9:e97649
Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC (2013) IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem 125:897–908
Kalonia H, Mishra J, Kumar A (2012) Targeting neuro-inflammatory cytokines and oxidative stress by minocycline attenuates quinolinic-acid-induced Huntington’s disease-like symptoms in rats. Neurotox Res 22(4):310–320
Yan EB, Frugier T, Lim CK, Heng B, Sundaram G, Tan M, Rosenfeld JV, Walker DW, Guillemin GJ, Morganti-Kossmann MC (2015) Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acidfollowing traumatic brain injury in humans. J Neuroinflammation 12:110
Whetsell Jr WO, Schwarcz R (1989) Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci Lett 97(3):271–275
Kerr SJ, Armati PJ, Guillemin GJ, Brew BJ (1998) Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. AIDS 12(4):355–363
Sei Y, Fossom L, Goping G, Skolnick P, Basile AS (1998) Quinolinic acid protects rat cerebellar granule cells from glutamate-induced apoptosis. Neurosci Lett 241(2–3):180–184
Obrenovitch TP (2001) Quinolinic acid accumulation during neuroinflammation. Does it imply excitotoxicity? Ann N Y Acad Sci 939:1–10
Brewer GJ, Torricelli JR (2007) Isolation and culture of adult neurons and neurospheres. Nat Protoc 2:1490–1498
Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, Chase TN (1999) Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 19:4023–4033
Levivier M, Przedborski S (1998) Quinolinic acid-induced lesions of the rat striatum: quantitative autoradiographic binding assessment. Neurol Res 20:46–56
Kim CH, Kim JH, Xu J, Hsu CY, Ahn YS (1999) Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J Neurochem 72:1586–1592
Nikodemova M, Duncan ID, Watters JJ (2006) Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem 96:314–323
Jiang X, Zhu D, Okagaki P, Lipsky R, Wu X, Banaudha K, Mearow K, Strauss KI, Marini AM (2003) N-methyl-D-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Ann N Y Acad Sci 993:134–145 (discussion 159–160)
Del Rosario JS, Feldmann KG, Ahmed T, Amjad U, Ko B, An J, Mahmud T, Salama M, Mei S, Asemota D, Mano I (2015) Death Associated Protein Kinase (DAPK) -mediated neurodegenerative mechanisms in nematodeexcitotoxicity. BMC Neurosci 16:25
Heyes MP, Saito K, Chen CY, Proescholdt MG, Nowak TS Jr, Li J, Beagles KE, Proescholdt MA, Zito MA, Kawai K, Markey SP (1997) Species heterogeneity between gerbils and rats: quinolinate production by microglia and astrocytes and accumulations in response to ischemic brain injury and systemic immune activation. J Neurochem 69(4):1519–1529
Heyes MP, Chen CY, Major EO, Saito K (1997) Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J 326(Pt 2):351–356
Frasca A, Aalbers M, Frigerio F, Fiordaliso F, Salio M, Gobbi M, Cagnotto A, Gardoni F, Battaglia GS, Hoogland G, Di Luca M, Vezzani A (2011) Misplaced NMDA receptors in epileptogenesis contribute to excitotoxicity. Neurobiol Dis 43(2):507–515
Wang Y, Wang W, Li D, Li M, Wang P, Wen J, Liang M, Su B, Yin Y (2014) IGF-1 alleviates NMDA-induced excitotoxicity in cultured hippocampal neurons against autophagy via the NR2B/PI3K-AKT-mTOR pathway. J Cell Physiol 229(11):1618–1629
Zhou X, Ding Q, Chen Z, Yun H, Wang H (2013) Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J Biol Chem 288(33):24151–24159
Lim CK, Fernández-Gomez FJ, Braidy N, Estrada C, Costa C, Costa S, Bessede A, Fernandez-Villalba E, Zinger A, Herrero MT, Guillemin GJ (2016) Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog Neurobiol S0301-0082(15): 30055–30061
Guillemin GJ, Meininger V, Brew BJ (2005) Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis 2:166–176
Pierozan P, Fernandes CG, Dutra MF, Pandolfo P, Ferreira F, de Lima BO, Porciúncula L, Wajner M, Pessoa-Pureur R (2014) Biochemical, histopathological and behavioral alterations caused by intrastriatal administration of quinolic acidto young rats. FEBS J 281:2061–2073
Vandresen-Filho S, Severino PC, Constantino LC, Martins WC, Molz S, Dal-Cim T, Bertoldo DB, Silva FR, Tasca CI (2015) N-methyl-D-aspartate preconditioning prevents quinolinic acid-induced deregulation of glutamate and calcium homeostasis in mice hippocampus. Neurotox Res 27:118–128
Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y (2010) Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia inmouse brain microvascular endothelial cells. J Pharmacol Sci 112(2):251–254
Qin ZH, Wang Y, Nakai M, Chase TN (1998) Nuclear factor-kappa B contributes to excitotoxin-induced apoptosis in rat striatum. Mol Pharmacol 53:33–42
Jin X, Yamashita T (2016) Microglia in central nervous system repair after injury. J Biochem 159:491–496
Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H (2016) Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci 36:577–589
Liu XX, Wang C, Huang SF, Chen Q, Hu YF, Zhou L, Gu Y (2016) Regnase-1 in microglia negatively regulates high mobility group box 1-mediated inflammation and neuronalinjury. Sci Rep 6:24073
Southam KA, Vincent AJ, Small DH (2016) Do microglia default on network maintenance in Alzheimer’s disease? J Alzheimers Dis 51:657–669
Abudara V, Roux L, Dallérac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C (2015) Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia 63:795–811
Ahlers KE, Karaçay B, Fuller L, Bonthius DJ, Dailey ME (2015) Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia 63:1694–1713
Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA (2005) Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia. Glia 50:21–31
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136
Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Chen SY, Raung SL, Lai CY (2012) Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-α signaling and contributes to neuronal death. Glia 60:487–501
Chen SH, Oyarzabal EA, Sung YF, Chu CH, Wang Q, Chen SL, Lu RB, Hong JS (2015) Microglial regulation of immunological and neuroprotective functions of astroglia. Glia 63:118–131
Kovacic P, Somanathan R (2010) Clinical physiology and mechanism of dizocilpine (MK-801): electron transfer, radicals, redox metabolites and bioactivity. Oxid Med Cell Longev 3:13–22
Lipton SA (2004) Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 1:101–110
Misztal M, Frankiewicz T, Parsons CG, Danysz W (1996) Learning deficits induced by chronic intraventricular infusion of quinolinic acid-protection by MK-801 and memantine. Eur J Pharmacol 296:1–8
Van der Staay FJ, Rutten K, Erb C, Blokland A (2011) Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res 220:215–229
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81671252; 81000547; 30930035), the National key basic science project 973 project (No. 2011CB510003) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Feng and Yan Wang contributed to this work equally.
Rights and permissions
About this article
Cite this article
Feng, W., Wang, Y., Liu, ZQ. et al. Microglia activation contributes to quinolinic acid-induced neuronal excitotoxicity through TNF-α. Apoptosis 22, 696–709 (2017). https://doi.org/10.1007/s10495-017-1363-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1363-5