Abstract
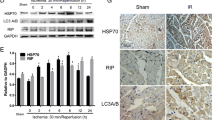
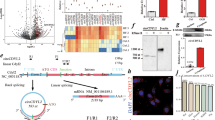
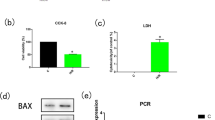
Myocardial ischemia and reperfusion (MIR) results in cardiomyocyte apoptosis with severe outcomes, which blocks cardiac tissue recovering from myocardial ischemia diseases. Heat shock protein 70 (HSP70) is one of protective molecule chaperones which could regulate the nucleus translocation of other proteins. In addition, eukaryotic elongation factor 2 (eEF2), which modulates protein translation process, is vital to the recovery of heart during MIR. However, the relationship between HSP70 and eEF2 and its effects on MIR are unclear. The expression and relationship between HSP70 and eEF2 is confirmed by western blot, immunoprecipitation in vitro using cardiomyocyte cell line H9c2 and in vivo rat MIR model. The further investigation was conducted in H9c2 cells with detection for cell-cycle and apoptosis. It is revealed that eEF2 interacted and be regulated by HSP70, which kept eEF2 as dephosphorylated status and preserved the function of eEF2 during MIR. In addition, HSP70 suppressed the nucleus translocation of phosphorylated eEF2, which inhibited cardiomyocyte apoptosis during myocardial reperfusion stage. Furthermore, HSP70 also interacted with C-terminal fragment of eEF2, which could reverse the nucleus translocation and cardiomyocyte apoptosis caused by N-terminal fragment of eEF2. HSP70 draw on advantage and avoid defect of MIR through regulating phosphorylation and nucleus translocation of eEF2.











Similar content being viewed by others
References
Wassenaar JW, Gaetani R, Garcia JJ, Braden RL, Luo CG, Huang D, DeMaria AN, Omens JH, Christman KL (2016) Evidence for mechanisms underlying the functional benefits of a myocardial matrix hydrogel for post-MI treatment. J Am Coll Cardiol 67(9):1074–1086. doi:10.1016/j.jacc.2015.12.035
Dharmakumar R (2015) Building a unified mechanistic insight into the bimodal pattern of edema in reperfused acute myocardial infarctions: observations, interpretations, and outlook. J Am Coll Cardiol 66(7):829–831. doi:10.1016/j.jacc.2015.05.074
Eisenhardt SU, Weiss JB, Smolka C, Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt Y, Bjoern Stark G, Moser M, Bode C, Grundmann S (2015) MicroRNA-155 aggravates ischemia-reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res Cardiol 110(3):32. doi:10.1007/s00395-015-0490-9
Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME (2012) CaMKII determines mitochondrial stress responses in heart. Nature 491(7423):269–273. doi:10.1038/nature11444
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL (1994) Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94(4):1621–1628. doi:10.1172/JCI117504
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44. doi:10.1146/annurev.physiol.010908.163111
Moludi J, Keshavarz S, Tabaee AS, Safiri S, Pakzad R (2016) Q10 supplementation effects on cardiac enzyme CK-MB and troponin in patients undergoing coronary artery bypass graft: a randomized, double-blinded, placebo-controlled clinical trial. J Cardiovasc Thorac Res 8(1):1–7. doi:10.15171/jcvtr.2016.01
Gottlieb RA, Finley KD, Mentzer RM Jr (2009) Cardioprotection requires taking out the trash. Basic Res Cardiol 104(2):169–180. doi:10.1007/s00395-009-0011-9
Salloum FN (2015) Hydrogen sulfide and cardioprotection–Mechanistic insights and clinical translatability. Pharmacol Ther 152:11–17. doi:10.1016/j.pharmthera.2015.04.004
Kim AS, Miller EJ, Wright TM, Li J, Qi D, Atsina K, Zaha V, Sakamoto K, Young LH (2011) A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol 51(1):24–32. doi:10.1016/j.yjmcc.2011.03.003
Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M, Agnihotri S, El-Naggar A, Yu B, Somasekharan SP, Faubert B, Bridon G, Tognon CE, Mathers J, Thomas R, Li A, Barokas A, Kwok B, Bowden M, Smith S, Wu X, Korshunov A, Hielscher T, Northcott PA, Galpin JD, Ahern CA, Wang Y, McCabe MG, Collins VP, Jones RG, Pollak M, Delattre O, Gleave ME, Jan E, Pfister SM, Proud CG, Derry WB, Taylor MD, Sorensen PH (2013) The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153(5):1064–1079. doi:10.1016/j.cell.2013.04.055
Kaul G, Pattan G, Rafeequi T (2011) Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct 29(3):227–234. doi:10.1002/cbf.1740
Crozier SJ, Vary TC, Kimball SR, Jefferson LS (2005) Cellular energy status modulates translational control mechanisms in ischemic-reperfused rat hearts. Am J Physiol Heart Circ Physiol 289(3):H1242–H1250. doi:10.1152/ajpheart.00859.2004
Yao Q, Liu BQ, Li H, McGarrigle D, Xing BW, Zhou MT, Wang Z, Zhang JJ, Huang XY, Guo L (2014) C-terminal Src kinase (Csk)-mediated phosphorylation of eukaryotic elongation factor 2 (eEF2) promotes proteolytic cleavage and nuclear translocation of eEF2. J Biol Chem 289(18):12666–12678. doi:10.1074/jbc.M113.546481
Bayram F, Bitgen N, Donmez-Altuntas H, Cakir I, Hamurcu Z, Sahin F, Simsek Y, Baskol G (2014) Increased genome instability and oxidative DNA damage and their association with IGF-1 levels in patients with active acromegaly. growth hormone & IGF research : official journal of the growth hormone research society and the International IGF Research. Society 24(1):29–34. doi:10.1016/j.ghir.2013.12.002
Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, Jones C, Radulescu S, Huels DJ, Myant KB, Dudek KM, Casey HA, Scopelliti A, Cordero JB, Vidal M, Pende M, Ryazanov AG, Sonenberg N, Meyuhas O, Hall MN, Bushell M, Willis AE, Sansom OJ (2015) mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature 517(7535):497–500. doi:10.1038/nature13896
Chen Z, Gopalakrishnan SM, Bui MH, Soni NB, Warrior U, Johnson EF, Donnelly JB, Glaser KB (2011) 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2): a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor. J Biol Chem 286(51):43951–43958. doi:10.1074/jbc.M111.301291
Iizuka A, Sengoku K, Iketani M, Nakamura F, Sato Y, Matsushita M, Nairn AC, Takamatsu K, Goshima Y, Takei K (2007) Calcium-induced synergistic inhibition of a translational factor eEF2 in nerve growth cones. Biochem Biophys Res Commun 353(2):244–250. doi:10.1016/j.bbrc.2006.11.150
Yu N, Kakunda M, Pham V, Lill JR, Du P, Wongchenko M, Yan Y, Firestein R, Huang X (2015) HSP105 recruits protein phosphatase 2 A to dephosphorylate beta-catenin. Mol Cell Biol 35(8):1390–1400. doi:10.1128/MCB.01307-14
Connarn JN, Assimon VA, Reed RA, Tse E, Southworth DR, Zuiderweg ER, Gestwicki JE, Sun D (2014) The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J Biol Chem 289(5):2908–2917. doi:10.1074/jbc.M113.519421
Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM (2015) Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65(15):1525–1536. doi:10.1016/j.jacc.2015.02.026
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell cycle 5(22):2592–2601
Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, Pasha Z, Wen Z, Rao F, Modi RM, Yu X, Ashraf M (2014) Heat shock improves Sca-1 + stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem cells 32(2):462–472. doi:10.1002/stem.1571
Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY (2000) Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stewss-induced inhibition of JNK dephosphorylation. J Biol Chem 275(48):38088–38094. doi:10.1074/jbc.M006632200
Choudhury S, Bae S, Ke Q, Lee JY, Kim J, Kang PM (2011) Mitochondria to nucleus translocation of AIF in mice lacking Hsp70 during ischemia/reperfusion. Basic Res Cardiol 106(3):397–407. doi:10.1007/s00395-011-0164-1
Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, Kouranti I, Spahr C, Pance A, Kroemer G, Garrido C (2003) Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 22(43):6669–6678. doi:10.1038/sj.onc.1206794
Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang L, Shen A (2012) Numbl inhibits glioma cell migration and invasion by suppressing TRAF5-mediated NF-kappaB activation. Mol Biol Cell 23(14):2635–2644. doi:10.1091/mbc.E11-09-0805
Wang Y, Liu F, Mao F, Hang Q, Huang X, He S, Wang Y, Cheng C, Wang H, Xu G, Zhang T, Shen A (2013) Interaction with cyclin H/cyclin-dependent kinase 7 (CCNH/CDK7) stabilizes C-terminal binding protein 2 (CtBP2) and promotes cancer cell migration. J Biol Chem 288(13):9028–9034. doi:10.1074/jbc.M112.432005
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J, Gu J (2012) Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity. Neurochem Int 61(7):1021–1035. doi:10.1016/j.neuint.2012.07.019
Wang J, Li Y, Liu Y, Li Y, Gong S, Fang F, Wang Z (2015) Overexpression of truncated AIF regulated by Egr1 promoter radiation-induced apoptosis on MCF-7 cells. Radiat Environ Biophys 54(4):413–421. doi:10.1007/s00411-015-0619-0
Wan C, Hou S, Ni R, Lv L, Ding Z, Huang X, Hang Q, He S, Wang Y, Cheng C, Gu XX, Xu G, Shen A (2015) MIF4G domain containing protein regulates cell cycle and hepatic carcinogenesis by antagonizing CDK2-dependent p27 stability. Oncogene 34(2):237–245. doi:10.1038/onc.2013.536
Lu D, Qian J, Li W, Feng Q, Pan S, Zhang S (2015) beta-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett 10(6):3434–3442. doi:10.3892/ol.2015.3769
Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M (2015) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta 1853(2):276–284. doi:10.1016/j.bbamcr.2014.11.015
Ranjani J, Pushpanathan M, Mahesh A, Niraimathi M, Gunasekaran P, Rajendhran J (2015) Pseudomonas aeruginosa PAO1 induces distinct cell death mechanisms in H9C2 cells and its differentiated form. J Basic Microbiol 55(10):1191–1202. doi:10.1002/jobm.201500037
Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH (2002) Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation 106 (12 Suppl 1):I270–1276
Sun L, Fan H, Yang L, Shi L, Liu Y (2015) Tyrosol prevents ischemia/reperfusion-induced cardiac injury in H9c2 cells: involvement of ROS, Hsp70, JNK and ERK, and apoptosis. Molecules 20(3):3758–3775. doi:10.3390/molecules20033758 pii]
Hafren A, Hofius D, Ronnholm G, Sonnewald U, Makinen K (2010) HSP70 and its cochaperone CPIP promote potyvirus infection in Nicotiana benthamiana by regulating viral coat protein functions. Plant Cell 22(2):523–535. doi:10.1105/tpc.109.072413
Gao T, Newton AC (2002) The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem 277(35):31585–31592. doi:10.1074/jbc.M204335200
Gao T, Newton AC (2006) Invariant Leu preceding turn motif phosphorylation site controls the interaction of protein kinase C with Hsp70. J Biol Chem 281(43):32461–32468. doi:10.1074/jbc.M604076200
Wullner U, Neef I, Eller A, Kleines M, Tur MK, Barth S (2008) Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr Cancer Drug Targets 8(7):554–565
Gismondi A, Caldarola S, Lisi G, Juli G, Chellini L, Iadevaia V, Proud CG, Loreni F (2014) Ribosomal stress activates eEF2K-eEF2 pathway causing translation elongation inhibition and recruitment of terminal oligopyrimidine (TOP) mRNAs on polysomes. Nucleic Acids Res 42(20):12668–12680. doi:10.1093/nar/gku996
Mazelin L, Panthu B, Nicot AS, Belotti E, Tintignac L, Teixeira G, Zhang Q, Risson V, Baas D, Delaune E, Derumeaux G, Taillandier D, Ohlmann T, Ovize M, Gangloff YG, Schaeffer L (2016) mTOR inactivation in myocardium from infant mice rapidly leads to dilated cardiomyopathy due to translation defects and p53/JNK-mediated apoptosis. J Mol Cell Cardiol. doi:10.1016/j.yjmcc.2016.04.011
Heise C, Taha E, Murru L, Ponzoni L, Cattaneo A, Guarnieri FC, Montani C, Mossa A, Vezzoli E, Ippolito G, Zapata J, Barrera I, Ryazanov AG, Cook J, Poe M, Stephen MR, Kopanitsa M, Benfante R, Rusconi F, Braida D, Francolini M, Proud CG, Valtorta F, Passafaro M, Sala M, Bachi A, Verpelli C, Rosenblum K, Sala C (2016) eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cereb Cortex. doi:10.1093/cercor/bhw075
Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H (2005) AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25(21):9554–9575. doi:10.1128/MCB.25.21.9554-9575.2005
Chen CY, Fang HY, Chiou SH, Yi SE, Huang CY, Chiang SF, Chang HW, Lin TY, Chiang IP, Chow KC (2011) Sumoylation of eukaryotic elongation factor 2 is vital for protein stability and anti-apoptotic activity in lung adenocarcinoma cells. Cancer Sci 102(8):1582–1589. doi:10.1111/j.1349-7006.2011.01975.x
Zhao Q, Xie Y, Zheng Y, Jiang S, Liu W, Mu W, Liu Z, Zhao Y, Xue Y, Ren J (2014) GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucl Acids Res 42 (Web Server issue):W325–330. doi:10.1093/nar/gku383
Sedek M, Strous GJ (2013) SUMOylation is a regulator of the translocation of Jak2 between nucleus and cytosol. Biochem J 453(2):231–239. doi:10.1042/BJ20121375
Ramakrishnan V, Davies C, Gerchman SE, Golden BL, Hoffmann DW, Jaishree TN, Kyila JH, Porter S, White SW (1995) Structures of prokaryotic ribosomal proteins: implications for RNA binding and evolution. Biochemistry Cell Biol 73 (11–12):979–986
Botos I, Melnikov EE, Cherry S, Tropea JE, Khalatova AG, Rasulova F, Dauter Z, Maurizi MR, Rotanova TV, Wlodawer A, Gustchina A (2004) The catalytic domain of Escherichia coli Lon protease has a unique fold and a Ser-Lys dyad in the active site. J Biol Chem 279(9):8140–8148. doi:10.1074/jbc.M312243200
Andreassi JL 2nd, Leyh TS (2004) Molecular functions of conserved aspects of the GHMP kinase family. BioChemistry 43(46):14594–14601. doi:10.1021/bi048963o
Ul-Haq Z, Gul S, Usmani S, Wadood A, Khan W (2015) Binding site identification and role of permanent water molecule of PIM-3 kinase: a molecular dynamics study. J Mol Gr Model 62:276–282. doi:10.1016/j.jmgm.2015.07.004
Ko SK, Kim J, Na DC, Park S, Park SH, Hyun JY, Baek KH, Kim ND, Kim NK, Park YN, Song K, Shin I (2015) A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem Biol 22 (3):391–403. doi:10.1016/j.chembiol.2015.02.004
Lin D, Tatham MH, Yu B, Kim S, Hay RT, Chen Y (2002) Identification of a substrate recognition site on Ubc9. J Biol Chem 277(24):21740–21748. doi:10.1074/jbc.M108418200
Kolesar P, Altmannova V, Silva S, Lisby M, Krejci L (2016) Pro-recombination role of Srs2 protein requires SUMO (Small Ubiquitin-like Modifier) but Is independent of PCNA (Proliferating Cell Nuclear Antigen) Interaction. J Biol Chem 291(14):7594–7607. doi:10.1074/jbc.M115.685891
Yang SH, Sharrocks AD (2010) The SUMO E3 ligase activity of Pc2 is coordinated through a SUMO interaction motif. Mol Cell Biol 30(9):2193–2205. doi:10.1128/MCB.01510-09
Wen D, Xu Z, Xia L, Liu X, Tu Y, Lei H, Wang W, Wang T, Song L, Ma C, Xu H, Zhu W, Chen G, Wu Y (2014) Important role of SUMOylation of spliceosome factors in prostate cancer cells. J Proteome Res 13(8):3571–3582. doi:10.1021/pr4012848
Ogawa S, Oishi H, Mezaki Y, Kouzu-Fujita M, Matsuyama R, Nakagomi M, Mori E, Murayama E, Nagasawa H, Kitagawa H, Yanagisawa J, Yano T, Kato S (2005) Repressive domain of unliganded human estrogen receptor alpha associates with Hsc70. Genes Cells 10 (12):1095–1102. doi:10.1111/j.1365-2443.2005.00904.x
Bottermann K, Reinartz M, Barsoum M, Kotter S, Godecke A (2013) Systematic analysis reveals elongation factor 2 and alpha-enolase as novel interaction partners of AKT2. PloS ONE 8(6):e66045. doi:10.1371/journal.pone.0066045
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, No. 2012CB822104); National Natural Science Foundation of China (Nos. 81401365, 31500647, 81171140, 81471258, 31440037 and 31270802) The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (14KJB180018); Nantong science and technology project (MS12015056, MS12015067); a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No competing financial interests exist.
Additional information
Chao Zhang, Xiaojuan Liu, Xiang Wu and Aiguo Shen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, C., Liu, X., Miao, J. et al. Heat shock protein 70 protects cardiomyocytes through suppressing SUMOylation and nucleus translocation of phosphorylated eukaryotic elongation factor 2 during myocardial ischemia and reperfusion. Apoptosis 22, 608–625 (2017). https://doi.org/10.1007/s10495-017-1355-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1355-5