Abstract
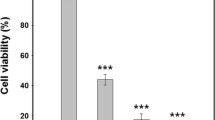
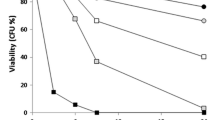
We have previously shown that the antifungal activity of human lactoferrin (hLf) against Candida albicans relies on its ability to induce cell death associated with apoptotic markers. To gain a deeper understanding of the mechanisms underlying hLf-induced apoptosis, we characterized this cell death process in the well-established Saccharomyces cerevisiae model. Our results indicate that hLf induces cell death in S. cerevisiae in a manner that requires energy and de novo protein synthesis. Cell death is associated with nuclear chromatin condensation, preservation of plasma membrane integrity, and is Yca1p metacaspase-dependent. Lactoferrin also caused mitochondrial dysfunction associated with ROS accumulation and release of cytochrome c. Pre-incubation with oligomycin, an oxidative phosphorylation inhibitor, increased resistance to hLf and, accordingly, mutants deficient in the F1F0-ATP synthase complex were more resistant to death induced by hLf. This indicates that mitochondrial energetic metabolism plays a key role in the killing effect of hLf, though a direct role of F1F0-ATP synthase cannot be precluded. Overexpression of the anti-apoptotic protein Bcl-xL or pre-incubation with N-acetyl cysteine reduced the intracellular level of ROS and increased resistance to hLf, confirming a ROS-mediated mitochondrial cell death process. Mitochondrial involvement was further reinforced by the higher resistance of cells lacking mitochondrial DNA, or other known yeast mitochondrial apoptosis regulators, such as, Aif1p, Cyc3p and Aac1/2/3p. This study provides new insights into a detailed understanding at the molecular level of hLf-induced apoptosis, which may allow the design of new strategies to overcome the emergence of resistance of clinically relevant fungi to conventional antifungals.




Similar content being viewed by others
References
Garcia-Montoya I, Gonzalez-Chavez SA, Salazar-Martinez J, Arevalo-Gallegos S, Sinagawa-Garcia S, Rascon-Cruz Q (2013) Expression and characterization of recombinant bovine lactoferrin in E. coli. Biometals 26(1):113–122. doi:10.1007/s10534-012-9598-7
Vogel HJ (2012) Lactoferrin, a bird’s eye view. Biochem Cell Biol 90(3):233–244. doi:10.1139/o2012-016
Lahoz E, Pisacane A, Iannaccone M, Palumbo D, Capparelli R (2008) Fungistatic activity of iron-free bovin lactoferrin against several fungal plant pathogens and antagonists. Nat Prod Res 22(11):955–961. doi:10.1080/14786410701650253
Wakabayashi H, Uchida K, Yamauchi K, Teraguchi S, Hayasawa H, Yamaguchi H (2000) Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J Antimicrob Chemother 46(4):595–602
Wang J, Xia XM, Wang HY, Li PP, Wang KY (2013) Inhibitory effect of lactoferrin against gray mould on tomato plants caused by Botrytis cinerea and possible mechanisms of action. Int J Food Microbiol 161(3):151–157. doi:10.1016/j.ijfoodmicro.2012.11.025
Al-Sheikh H (2009) Effect of lactoferrin and iron on the growth of human pathogenic Candida species. Pak J Biol Sci 12(1):91–94
Xu YY, Samaranayake YH, Samaranayake LP, Nikawa H (1999) In vitro susceptibility of Candida species to lactoferrin. Med Mycol 37(1):35–41
Viejo-Diaz M, Andres MT, Fierro JF (2004) Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extracellular cation concentration and target cell metabolic activity. Antimicrob Agents Chemother 48(4):1242–1248
Andres MT, Viejo-Diaz M, Fierro JF (2008) Human lactoferrin induces apoptosis-like cell death in Candida albicans: critical role of K+ -channel-mediated K+ efflux. Antimicrob Agents Chemother 52(11):4081–4088. doi:10.1128/AAC.01597-07
Pereira C, Silva RD, Saraiva L, Johansson B, Sousa MJ, Corte-Real M (2008) Mitochondria-dependent apoptosis in yeast. Biochim Biophys Acta 1783(7):1286–1302. doi:10.1016/j.bbamcr.2008.03.010
Carmona-Gutierrez D, Eisenberg T, Buttner S, Meisinger C, Kroemer G, Madeo F (2010) Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ 17(5):763–773. doi:10.1038/cdd.2009.219
Sousa M, Duarte AM, Fernandes TR, Chaves SR, Pacheco A, Leao C, Corte-Real M, Sousa MJ (2013) Genome-wide identification of genes involved in the positive and negative regulation of acetic acid-induced programmed cell death in Saccharomyces cerevisiae. BMC Genom 14:838. doi:10.1186/1471-2164-14-838
Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Corte-Real M (2002) Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol Biol Cell 13(8):2598–2606. doi:10.1091/mbc.E01-12-0161
Madeo F, Engelhardt S, Herker E, Lehmann N, Maldener C, Proksch A, Wissing S, Frohlich KU (2002) Apoptosis in yeast: a new model system with applications in cell biology and medicine. Curr Genet 41(4):208–216. doi:10.1007/s00294-002-0310-2
Ludovico P, Madeo F, Silva M (2005) Yeast programmed cell death: an intricate puzzle. IUBMB Life 57(3):129–135. doi:10.1080/15216540500090553
Frohlich KU, Fussi H, Ruckenstuhl C (2007) Yeast apoptosis-from genes to pathways. Semin Cancer Biol 17(2):112–121. doi:10.1016/j.semcancer.2006.11.006
Gietz RD, Woods RA (2006) Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol Biol 313:107–120. doi:10.1385/1-59259-958-3:107
Rego A, Costa M, Chaves SR, Matmati N, Pereira H, Sousa MJ, Moradas-Ferreira P, Hannun YA, Costa V, Corte-Real M (2012) Modulation of mitochondrial outer membrane permeabilization and apoptosis by ceramide metabolism. PLoS One 7(11):e48571. doi:10.1371/journal.pone.0048571
Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Frohlich KU (1999) Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol 145(4):757–767
O’Donnell AF, McCartney RR, Chandrashekarappa DG, Zhang BB, Thorner J, Schmidt MC (2015) 2-Deoxyglucose impairs Saccharomyces cerevisiae growth by stimulating Snf1-regulated and alpha-arrestin-mediated trafficking of hexose transporters 1 and 3. Mol Cell Biol 35(6):939–955. doi:10.1128/MCB.01183-14
Madeo F, Herker E, Maldener C, Wissing S, Lachelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Frohlich KU (2002) A caspase-related protease regulates apoptosis in yeast. Mol Cell 9(4):911–917
Sun SY (2010) N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther 9(2):109–110
Dumont ME, Cardillo TS, Hayes MK, Sherman F (1991) Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol Cell Biol 11(11):5487–5496
Wissing S, Ludovico P, Herker E, Buttner S, Engelhardt SM, Decker T, Link A, Proksch A, Rodrigues F, Corte-Real M, Frohlich KU, Manns J, Cande C, Sigrist SJ, Kroemer G, Madeo F (2004) An AIF orthologue regulates apoptosis in yeast. J Cell Biol 166(7):969–974. doi:10.1083/jcb.200404138
Eisenberg T, Buttner S, Kroemer G, Madeo F (2007) The mitochondrial pathway in yeast apoptosis. Apoptosis 12(5):1011–1023. doi:10.1007/s10495-007-0758-0
Ackerman SH, Tzagoloff A (1990) ATP10, a yeast nuclear gene required for the assembly of the mitochondrial F1-F0 complex. J Biol Chem 265(17):9952–9959
Pereira C, Camougrand N, Manon S, Sousa MJ, Corte-Real M (2007) ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol Microbiol 66(3):571–582. doi:10.1111/j.1365-2958.2007.05926.x
Slonimski PP, Perrodin G, Croft JH (1968) Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal “petites”. Biochem Biophys Res Commun 30(3):232–239
Kuwana T, Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15(6):691–699
Mazzoni C, Falcone C (2008) Caspase-dependent apoptosis in yeast. Biochim Biophys Acta 1783(7):1320–1327. doi:10.1016/j.bbamcr.2008.02.015
Vachova L, Palkova Z (2007) Caspases in yeast apoptosis-like death: facts and artefacts. FEMS Yeast Res 7(1):12–21. doi:10.1111/j.1567-1364.2006.00137.x
Pearce DA, Sherman F (1995) Degradation of cytochrome oxidase subunits in mutants of yeast lacking cytochrome c and suppression of the degradation by mutation of yme1. J Biol Chem 270(36):20879–20882
Epstein CB, Waddle JA, Wt Hale, Dave V, Thornton J, Macatee TL, Garner HR, Butow RA (2001) Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell 12(2):297–308
Rasola A, Bernardi P (2007) The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12(5):815–833. doi:10.1007/s10495-007-0723-y
Scharstuhl A, Mutsaers HA, Pennings SW, Russel FG, Wagener FA (2009) Involvement of VDAC, Bax and ceramides in the efflux of AIF from mitochondria during curcumin-induced apoptosis. PLoS One 4(8):e6688. doi:10.1371/journal.pone.0006688
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12(5):835–840. doi:10.1007/s10495-006-0525-7
Zhivotovsky B, Galluzzi L, Kepp O, Kroemer G (2009) Adenine nucleotide translocase: a component of the phylogenetically conserved cell death machinery. Cell Death Differ 16(11):1419–1425. doi:10.1038/cdd.2009.118
Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P (2013) Role of the c subunit of the F0 ATP synthase in mitochondrial permeability transition. Cell Cycle 12(4):674–683. doi:10.4161/cc.23599
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34(12):1475–1486. doi:10.1038/onc.2014.96
Kissova I, Plamondon LT, Brisson L, Priault M, Renouf V, Schaeffer J, Camougrand N, Manon S (2006) Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by Bax expression in yeast. J Biol Chem 281(47):36187–36197. doi:10.1074/jbc.M607444200
Aucott JN, Fayen J, Grossnicklas H, Morrissey A, Lederman MM, Salata RA (1990) Invasive infection with Saccharomyces cerevisiae: report of three cases and review. Rev Infect Dis 12(3):406–411
Cimolai N, Gill MJ, Church D (1987) Saccharomyces cerevisiae fungemia: case report and review of the literature. Diagn Microbiol Infect Dis 8(2):113–117
Enache-Angoulvant A, Hennequin C (2005) Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis 41(11):1559–1568. doi:10.1086/497832
Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Magwene PM, Dietrich FS, McCusker JH (2015) The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res 25(5):762–774. doi:10.1101/gr.185538.114
Kobayashi T, Kakeya H, Miyazaki T, Izumikawa K, Yanagihara K, Ohno H, Yamamoto Y, Tashiro T, Kohno S (2011) Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. Jpn J Infect Dis 64(4):292–296
Lupetti A, Brouwer CP, Bogaards SJ, Welling MM, de Heer E, Campa M, van Dissel JT, Friesen RH, Nibbering PH (2007) Human lactoferrin-derived peptide’s antifungal activities against disseminated Candida albicans infection. J Infect Dis 196(9):1416–1424. doi:10.1086/522427
Mishra B, Leishangthem GD, Gill K, Singh AK, Das S, Singh K, Xess I, Dinda A, Kapil A, Patro IK, Dey S (1828) A novel antimicrobial peptide derived from modified N-terminal domain of bovine lactoferrin: design, synthesis, activity against multidrug-resistant bacteria and Candida. Biochim Biophys Acta 2:677–686. doi:10.1016/j.bbamem.2012.09.021
Cerqueira DF, Portela MB, Pomarico L, de Araujo Soares RM, de Souza IP, Castro GF (2010) Oral Candida colonization and its relation with predisposing factors in HIV-infected children and their uninfected siblings in Brazil: the era of highly active antiretroviral therapy. J Oral Pathol Med 39(2):188–194. doi:10.1111/j.1600-0714.2009.00857
Acknowledgments
This work was supported by the Ministerio de Educación, Programa Campus de Excelencia Internacional-Subprograma de Fortalecimiento (Convocatoria 2010) and the grant UNOV-10-BECDOC given to Maikel Acosta Zaldívar by University of Oviedo. This work was supported by Fundação para a Ciência e Tecnologia (FCT) through the strategic funding UID/BIA/04050/2013, and through the project Pest- FCT-ANR/BEX-BCM/0175/2012. A. Rego was the recipient of a FCT fellowship (SFRH/BD/79523/2011). C.S.Pereira was the recipient of a fellowship from the project FCT-ANR/BEX-BCM/0175/2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Acosta-Zaldívar, M., Andrés, M.T., Rego, A. et al. Human lactoferrin triggers a mitochondrial- and caspase-dependent regulated cell death in Saccharomyces cerevisiae . Apoptosis 21, 163–173 (2016). https://doi.org/10.1007/s10495-015-1199-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1199-9