Abstract
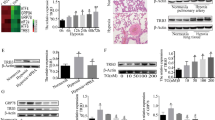
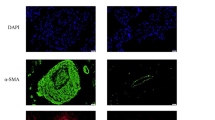
Pulmonary arterial hypertension (PAH) is a life-threatening disorder that ultimately causes heart failure. While the underlying causes of this condition are not well understood, previous studies suggest that the anti-apoptotic nature of pulmonary microvascular endothelial cells (PMVECs) in hypoxic environments contributes to PAH pathogenesis. In this study, we focus on the contribution of Bcl-2 and hypoxia response element (HRE) to apoptosis-resistant endothelial cells and investigate the mechanism. PMVECs obtained from either normal rats or apoptosis-resistant PMVECs obtained from PAH rats were transduced with recombinant lentiviral vectors carrying either Bcl-2-shRNA or HRE combined Bcl-2-shRNA, and then cultured these cells for 24 h under hypoxic (5 % O2) or normoxic (21 % O2) conditions. In normal PMVECs, Bcl-2-shRNA or HRE combined with Bcl-2-shRNA transduction successfully decreased Bcl-2 expression, while increasing apoptosis as well as caspase-3 and P53 expression in a normoxic environment. In a hypoxic environment, the effects of Bcl-2-shRNA treatment on cell apoptosis, and on Bcl-2, caspase-3, P53 expression were significantly suppressed. Conversely, HRE activation combined with Bcl-2-shRNA transduction markedly enhanced cell apoptosis and upregulated caspase-3 and P53 expression, while decreasing Bcl-2 expression. Furthermore, in apoptosis-resistant PMVECs, HRE-mediated Bcl-2 silencing effectively enhanced cell apoptosis and caspase-3 activity. The apoptosis rate was significantly depressed when Lv-HRE-Bcl-2-shRNA was combined with Lv-P53-shRNA or Lv-caspase3-shRNA transduction in a hypoxic environment. These results suggest that HRE-mediated Bcl-2 inhibition can effectively attenuate hypoxia-induced apoptosis resistance in PMVECs by downregulating Bcl-2 expression and upregulating caspase-3 and P53 expression. This study therefore reveals critical insight into potential therapeutic targets for treating PAH.










Similar content being viewed by others
References
Sugumaran PK, Wang S, Song S, Nie X, Zhang L, Feng Y, Ma W, Zhu D (2014) 15-Oxo-eicosatetraenoic acid prevents serum deprivation-induced apoptosis of pulmonary arterial smooth muscle cells by activating pro-survival pathway. Prostaglandins Leukot Essent Fat Acids 90(4):89–98. doi:10.1016/j.plefa.2014.01.006
Jiang J, Wang S, Wang Z, Ma J, Liu S, Li W, Zhu D (2011) The role of ERK1/2 in 15-HETE-inhibited apoptosis in pulmonary arterial smooth muscle cells. J Recep Signal Transduct Res 31(1):45–52. doi:10.3109/10799893.2010.512013
Huh JW, Kim SY, Lee JH, Lee YS (2011) YC-1 attenuates hypoxia-induced pulmonary arterial hypertension in mice. Pulm Pharmacol Ther 24(6):638–646. doi:10.1016/j.pupt.2011.09.003
Lee FY, Lu HI, Zhen YY, Leu S, Chen YL, Tsai TH, Chung SY, Chua S, Sheu JJ, Hsu SY, Chang HW, Sun CK, Yip HK (2013) Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur J Pharm Sci 50(3–4):372–384. doi:10.1016/j.ejps.2013.08.004
Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL (2006) An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113(22):2630–2641. doi:10.1161/circulationaha.105.609008
Jeffery TK, Morrell NW (2002) Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45(3):173–202. doi:10.1053/pcad.2002.130041
Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF (2005) Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19(9):1178–1180. doi:10.1096/fj.04-3261fje
Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX (2004) Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 68(2):75–103. doi:10.1016/j.mvr.2004.06.001
Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, Voelkel NF (2006) Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol 291(3):L362–L368. doi:10.1152/ajplung.00111.2005
Thomas HC, Lame MW, Dunston SK, Segall HJ, Wilson DW (1998) Monocrotaline pyrrole induces apoptosis in pulmonary artery endothelial cells. Toxicol Appl Pharmacol 151(2):236–244. doi:10.1006/taap.1998.8458
Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC (2007) Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293(3):L548–L554. doi:10.1152/ajplung.00428.2006
Nickel N, Jonigk D, Kempf T, Bockmeyer CL, Maegel L, Rische J, Laenger F, Lehmann U, Sauer C, Greer M, Welte T, Hoeper MM, Golpon HA (2011) GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res 12:62. doi:10.1186/1465-9921-12-62
Levy M, Maurey C, Celermajer DS, Vouhe PR, Danel C, Bonnet D, Israel-Biet D (2007) Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 49(7):803–810. doi:10.1016/j.jacc.2006.09.049
Ichimori H, Kogaki S, Takahashi K, Ishida H, Narita J, Nawa N, Baden H, Uchikawa T, Okada Y, Ozono K (2013) Drastic shift from positive to negative estrogen effect on bone morphogenetic protein signaling in pulmonary arterial endothelial cells under hypoxia. Circ J 77(8):2118–2126
Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF (2001) Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195(3):367–374. doi:10.1002/path.953
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70(5):1469–1480. doi:10.1124/mol.106.027029
Ruan H, Wang J, Hu L, Lin CS, Lamborn KR, Deen DF (1999) Killing of brain tumor cells by hypoxia-responsive element mediated expression of BAX. Neoplasia 1(5):431–437
Jiang B, Dong H, Zhang Z, Wang W, Zhang Y, Xu X (2007) Hypoxic response elements control expression of human vascular endothelial growth factor(165) genes transferred to ischemia myocardium in vivo and in vitro. J Gene Med 9(9):788–796. doi:10.1002/jgm.1070
Cheng C, Liu H, Ge H, Qian J, Qin J, Sun L, Chen M, Yan M, Shen A (2007) Lipopolysaccharide induces expression of SSeCKS in rat lung microvascular endothelial cell. Mol Cell Biochem 305(1–2):1–8. doi:10.1007/s11010-007-9521-7
Huang H, Zhang P, Wang Z, Tang F, Jiang Z (2011) Activation of endothelin-1 receptor signaling pathways is associated with neointima formation, neoangiogenesis and irreversible pulmonary artery hypertension in patients with congenital heart disease. Circ J 75(6):1463–1471
Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF (2006) Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 291(4):L668–L676. doi:10.1152/ajplung.00491.2005
Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, Ghofrani HA, Howard L, Nihoyannopoulos P, Mohiaddin RH, Gibbs JS (2010) Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med 181(10):1106–1113. doi:10.1164/rccm.2009111-699OC/rccm.200911-1699OC
Chen C, Chen C, Wang Z, Wang L, Yang L, Ding M, Ding C, Sun Y, Lin Q, Huang X, Du X, Zhao X, Wang C (2012) Puerarin induces mitochondria-dependent apoptosis in hypoxic human pulmonary arterial smooth muscle cells. PLoS One 7(3):e34181. doi:10.1371/journal.pone.0034181
Li HL, Huang XP, Zhou XH, Ji TH, Wu ZQ, Wang ZQ, Jiang HY, Liu FR, Zhao T (2011) Correlation of seven biological factors (Hsp90a, p53, MDM2, Bcl-2, Bax, Cytochrome C, and Cleaved caspase 3) with clinical outcomes of ALK+ anaplastic large-cell lymphoma. Biomed Environ Sci 24(6):630–641. doi:10.3967/0895-3988.2011.06.007
Sakao S, Taraseviciene-Stewart L, Lee JD (2005) Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19(9):1178–1796
Huang LE, Bunn HF (2003) Hypoxia-inducible factor and its biomedical relevance. J Biol Chem 278(22):19575–19578. doi:10.1074/jbc.R200030200
Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM (2010) Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176(3):1130–1138. doi:10.2353/ajpath.2010.090832
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12(12):5447–5454
Park J, Park SY, Shin E, Lee SH, Kim YS, Lee DH, Roh GS, Kim HJ, Kang SS, Cho GJ, Jeong BY, Kim H, Choi WS (2014) Hypoxia inducible factor-1alpha directly regulates nuclear clusterin transcription by interacting with hypoxia response elements in the clusterin promoter. Mol Cells 37(2):178–186. doi:10.14348/molcells.2014.2349
Ahn YT, Chua MS, Whitlock JP Jr, Shin YC, Song WH, Kim Y, Eom CY, An WG (2010) Rodent-specific hypoxia response elements enhance PAI-1 expression through HIF-1 or HIF-2 in mouse hepatoma cells. Int J Oncol 37(6):1627–1638
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71. doi:10.1146/annurev-pathol-012513-104720
Jeong CH, Lee YM, Choi KS, Seong YR, Kim YJ, Im DS, Kim KW (2005) Hypoxia-responsive element-mediated soluble Tie2 vector exhibits an anti-angiogenic activity in vitro under hypoxic condition. Int J Oncol 26(1):211–216
Horiuchi A, Hayashi T, Kikuchi N, Hayashi A, Fuseya C, Shiozawa T, Konishi I (2012) Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1alpha to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int J Cancer 131(8):1755–1767. doi:10.1002/ijc.27448
Fujioka T, Matsunaga N, Okazaki H, Koyanagi S, Ohdo S (2010) Hypoxia-response plasmid vector producing bcl-2 shRNA enhances the apoptotic cell death of mouse rectum carcinoma. J Pharmacol Sci 113(4):353–361
Segura MM, Mangion M, Gaillet B, Garnier A (2013) New developments in lentiviral vector design, production and purification. Exp Opin Biol Ther 13(7):987–1011. doi:10.1517/14712598.2013.779249
Acknowledgments
This work was supported by the Science and Technology Development Fund of Shanghai Pudong New Area (PKj2011-y36) and the National Nature Science Foundation of China (No. 81272145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
We declare that we have no conflict of interest.
Additional information
Yongmei Cao and Zhen Jiang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Cao, Y., Jiang, Z., Zeng, Z. et al. Bcl-2 silencing attenuates hypoxia-induced apoptosis resistance in pulmonary microvascular endothelial cells. Apoptosis 21, 69–84 (2016). https://doi.org/10.1007/s10495-015-1184-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1184-3