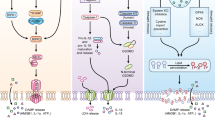
Abstract
Death receptors such as Tumor necrosis factor receptor 1, FAS and TNF-associated apoptosis-inducing ligand-R1/2 play a major role in counteracting with bacterial pathogen infection through regulation of inflammation and programmed cell death. The highly regulated death receptor signaling is frequently targeted by gram-negative bacterial pathogens such as Salmonella, Shigella, enteropathogenic Escherichia coli and enterohamorrhagic Escherichia coli, which harbor a conserved type III secretion system that delivers a repertoire of effector proteins to manipulate host signal transductions for their own benefit. This review focuses on how bacterial gut pathogens hijack death receptor signaling to inhibit host NF-κB and programmed cell death pathways.

Similar content being viewed by others
References
Park HH, Lo YC, Lin SC, Wang L, Yang JK, Wu H (2007) The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol 25:561–586. doi:10.1146/annurev.immunol.25.022106.141656
Ferrao R, Wu H (2012) Helical assembly in the death domain (DD) superfamily. Curr Opin Struct Biol 22(2):241–247. doi:10.1016/j.sbi.2012.02.006
Guicciardi ME, Gores GJ (2009) Life and death by death receptors. FASEB J 23(6):1625–1637. doi:10.1096/fj.08-111005
Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW (1993) Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73(3):457–467
Zhao YX, Lajoie G, Zhang H, Chiu B, Payne U, Inman RD (2000) Tumor necrosis factor receptor p55-deficient mice respond to acute Yersinia enterocolitica infection with less apoptosis and more effective host resistance. Infect Immun 68(3):1243–1251
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114(2):181–190
MacEwan DJ (2002) TNF ligands and receptors–a matter of life and death. Br J Pharmacol 135(4):855–875. doi:10.1038/sj.bjp.0704549
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137(6):1100–1111. doi:10.1016/j.cell.2009.05.021
Galan JE, Wolf-Watz H (2006) Protein delivery into eukaryotic cells by type III secretion machines. Nature 444(7119):567–573. doi:10.1038/nature05272
Coburn B, Sekirov I, Finlay BB (2007) Type III secretion systems and disease. Clin Microbiol Rev 20(4):535–549. doi:10.1128/CMR.00013-07
Bhavsar AP, Guttman JA, Finlay BB (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449(7164):827–834. doi:10.1038/nature06247
Roy CR, Mocarski ES (2007) Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol 8(11):1179–1187. doi:10.1038/ni1528
Cui J, Shao F (2011) Biochemistry and cell signaling taught by bacterial effectors. Trends Biochem Sci 36(10):532–540. doi:10.1016/j.tibs.2011.07.003
Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, Gao W, Ding X, Sun L, Chen X, Chen S, Shao F (2013) Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 501(7466):242–246. doi:10.1038/nature12436
Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, Wagstaff KM, Dunstone MA, Sloan J, Whisstock JC, Kaper JB, Robins-Browne RM, Jans DA, Frankel G, Phillips AD, Coulson BS, Hartland EL (2010) The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog 6(5):e1000898. doi:10.1371/journal.ppat.1000898
Brown NF, Coombes BK, Bishop JL, Wickham ME, Lowden MJ, Gal-Mor O, Goode DL, Boyle EC, Sanderson KL, Finlay BB (2011) Salmonella phage ST64B encodes a member of the SseK/NleB effector family. PLoS ONE 6(3):e17824. doi:10.1371/journal.pone.0017824
Kujat Choy SL, Boyle EC, Gal-Mor O, Goode DL, Valdez Y, Vallance BA, Finlay BB (2004) SseK1 and SseK2 are novel translocated proteins of Salmonella enterica serovar typhimurium. Infect Immun 72(9):5115–5125. doi:10.1128/IAI.72.9.5115-5125.2004
Kelly M, Hart E, Mundy R, Marches O, Wiles S, Badea L, Luck S, Tauschek M, Frankel G, Robins-Browne RM, Hartland EL (2006) Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect Immun 74(4):2328–2337. doi:10.1128/IAI.74.4.2328-2337.2006
Misyurina O, Asper DJ, Deng W, Finlay BB, Rogan D, Potter AA (2010) The role of Tir, EspA, and NleB in the colonization of cattle by Shiga toxin producing Escherichia coli O26:H11. Can J Microbiol 56(9):739–747. doi:10.1139/w10-059
Wickham ME, Lupp C, Mascarenhas M, Vazquez A, Coombes BK, Brown NF, Coburn BA, Deng W, Puente JL, Karmali MA, Finlay BB (2006) Bacterial genetic determinants of non-O157 STEC outbreaks and hemolytic-uremic syndrome after infection. J Infect Dis 194(6):819–827. doi:10.1086/506620
Gao X, Wang X, Pham TH, Feuerbacher LA, Lubos ML, Huang M, Olsen R, Mushegian A, Slawson C, Hardwidge PR (2013) NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-kappaB activation. Cell Host Microbe 13(1):87–99. doi:10.1016/j.chom.2012.11.010
Pearson JS, Giogha C, Ong SY, Kennedy CL, Kelly M, Robinson KS, Lung TW, Mansell A, Riedmaier P, Oates CV, Zaid A, Muhlen S, Crepin VF, Marches O, Ang CS, Williamson NA, O’Reilly LA, Bankovacki A, Nachbur U, Infusini G, Webb AI, Silke J, Strasser A, Frankel G, Hartland EL (2013) A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature 501(7466):247–251. doi:10.1038/nature12524
Zhang L, Ding X, Cui J, Xu H, Chen J, Gong YN, Hu L, Zhou Y, Ge J, Lu Q, Liu L, Chen S, Shao F (2012) Cysteine methylation disrupts ubiquitin-chain sensing in NF-kappaB activation. Nature 481(7380):204–208. doi:10.1038/nature10690
Ruchaud-Sparagano MH, Muhlen S, Dean P, Kenny B (2011) The enteropathogenic E. coli (EPEC) Tir effector inhibits NF-kappaB activity by targeting TNFalpha receptor-associated factors. PLoS Pathog 7(12):e1002414. doi:10.1371/journal.ppat.1002414
Yan D, Wang X, Luo L, Cao X, Ge B (2012) Inhibition of TLR signaling by a bacterial protein containing immunoreceptor tyrosine-based inhibitory motifs. Nat Immunol 13(11):1063–1071. doi:10.1038/ni.2417
Yan D, Quan H, Wang L, Liu F, Liu H, Chen J, Cao X, Ge B (2013) Enteropathogenic Escherichia coli Tir recruits cellular SHP-2 through ITIM motifs to suppress host immune response. Cell Signal 25(9):1887–1894. doi:10.1016/j.cellsig.2013.05.020
Terzic J, Marinovic-Terzic I, Ikeda F, Dikic I (2007) Ubiquitin signals in the NF-kappaB pathway. Biochem Soc Trans 35(Pt 5):942–945. doi:10.1042/BST0350942
Skaug B, Jiang X, Chen ZJ (2009) The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem 78:769–796. doi:10.1146/annurev.biochem.78.070907.102750
Nadler C, Baruch K, Kobi S, Mills E, Haviv G, Farago M, Alkalay I, Bartfeld S, Meyer TF, Ben-Neriah Y, Rosenshine I (2010) The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog 6(1):e1000743. doi:10.1371/journal.ppat.1000743
Vossenkamper A, Marches O, Fairclough PD, Warnes G, Stagg AJ, Lindsay JO, Evans PC, le Luong A, Croft NM, Naik S, Frankel G, MacDonald TT (2010) Inhibition of NF-kappaB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE. J Immunol 185(7):4118–4127. doi:10.4049/jimmunol.1000500
Meyer HH, Wang Y, Warren G (2002) Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J 21(21):5645–5652
Wang B, Alam SL, Meyer HH, Payne M, Stemmler TL, Davis DR, Sundquist WI (2003) Structure and ubiquitin interactions of the conserved zinc finger domain of Npl4. J Biol Chem 278(22):20225–20234. doi:10.1074/jbc.M300459200
Hsu Y, Jubelin G, Taieb F, Nougayrede JP, Oswald E, Stebbins CE (2008) Structure of the cyclomodulin Cif from pathogenic Escherichia coli. J Mol Biol 384(2):465–477. doi:10.1016/j.jmb.2008.09.051
Crow A, Race PR, Jubelin G, Varela Chavez C, Escoubas JM, Oswald E, Banfield MJ (2009) Crystal structures of Cif from bacterial pathogens Photorhabdus luminescens and Burkholderia pseudomallei. PLoS ONE 4(5):e5582. doi:10.1371/journal.pone.0005582
Yao Q, Cui J, Zhu Y, Wang G, Hu L, Long C, Cao R, Liu X, Huang N, Chen S, Liu L, Shao F (2009) A bacterial type III effector family uses the papain-like hydrolytic activity to arrest the host cell cycle. Proc Natl Acad Sci USA 106(10):3716–3721. doi:10.1073/pnas.0900212106
Cui J, Yao Q, Li S, Ding X, Lu Q, Mao H, Liu L, Zheng N, Chen S, Shao F (2010) Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 329(5996):1215–1218. doi:10.1126/science.1193844
Kim DW, Lenzen G, Page AL, Legrain P, Sansonetti PJ, Parsot C (2005) The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc Natl Acad Sci USA 102(39):14046–14051. doi:10.1073/pnas.0504466102
Zhou Y, Dong N, Hu L, Shao F (2013) The Shigella type three secretion system effector OspG directly and specifically binds to host ubiquitin for activation. PLoS ONE 8(2):e57558. doi:10.1371/journal.pone.0057558
Pruneda JN, Smith FD, Daurie A, Swaney DL, Villen J, Scott JD, Stadnyk AW, Le Trong I, Stenkamp RE, Klevit RE, Rohde JR, Brzovic PS (2014) E2 ~ Ub conjugates regulate the kinase activity of Shigella effector OspG during pathogenesis. EMBO J 33(5):437–449. doi:10.1002/embj.201386386
Grishin AM, Condos TE, Barber KR, Campbell-Valois FX, Parsot C, Shaw GS, Cygler M (2014) Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG. Structure 22(6):878–888. doi:10.1016/j.str.2014.04.010
Grishin AM, Cherney M, Anderson DH, Phanse S, Babu M, Cygler M (2014) NleH defines a new family of bacterial effector kinases. Structure 22(2):250–259. doi:10.1016/j.str.2013.11.006
Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, Deutsch WA, Lenardo MJ (2007) Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell 131(5):927–939. doi:10.1016/j.cell.2007.10.009
Gao X, Wan F, Mateo K, Callegari E, Wang D, Deng W, Puente J, Li F, Chaussee MS, Finlay BB, Lenardo MJ, Hardwidge PR (2009) Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog 5(12):e1000708. doi:10.1371/journal.ppat.1000708
Hemrajani C, Berger CN, Robinson KS, Marches O, Mousnier A, Frankel G (2010) NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc Natl Acad Sci USA 107(7):3129–3134. doi:10.1073/pnas.0911609106
Robinson KS, Mousnier A, Hemrajani C, Fairweather N, Berger CN, Frankel G (2010) The enteropathogenic Escherichia coli effector NleH inhibits apoptosis induced by Clostridium difficile toxin B. Microbiology 156(Pt 6):1815–1823. doi:10.1099/mic.0.037259-0
Ashida H, Toyotome T, Nagai T, Sasakawa C (2007) Shigella chromosomal IpaH proteins are secreted via the type III secretion system and act as effectors. Mol Microbiol 63(3):680–693. doi:10.1111/j.1365-2958.2006.05547.x
Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C (2010) A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat Cell Biol 12(1):66–73. doi:10.1038/ncb2006 61–69
Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, Yogev O, Shaulian E, Guttman C, Zarivach R, Rosenshine I (2011) Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. EMBO J 30(1):221–231. doi:10.1038/emboj.2010.297
Pearson JS, Riedmaier P, Marches O, Frankel G, Hartland EL (2011) A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-kappaB for degradation. Mol Microbiol 80(1):219–230. doi:10.1111/j.1365-2958.2011.07568.x
Sham HP, Shames SR, Croxen MA, Ma C, Chan JM, Khan MA, Wickham ME, Deng W, Finlay BB, Vallance BA (2011) Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-kappaB and p38 mitogen-activated protein kinase activation. Infect Immun 79(9):3552–3562. doi:10.1128/IAI.05033-11
Yen H, Ooka T, Iguchi A, Hayashi T, Sugimoto N, Tobe T (2010) NleC, a type III secretion protease, compromises NF-kappaB activation by targeting p65/RelA. PLoS Pathog 6(12):e1001231. doi:10.1371/journal.ppat.1001231
Muhlen S, Ruchaud-Sparagano MH, Kenny B (2011) Proteasome-independent degradation of canonical NFkappaB complex components by the NleC protein of pathogenic Escherichia coli. J Biol Chem 286(7):5100–5107. doi:10.1074/jbc.M110.172254
Turco MM, Sousa MC (2014) The structure and specificity of the type III secretion system effector NleC suggest a DNA mimicry mechanism of substrate recognition. Biochemistry 53(31):5131–5139. doi:10.1021/bi500593e
Wickham ME, Brown NF, Boyle EC, Coombes BK, Finlay BB (2007) Virulence is positively selected by transmission success between mammalian hosts. Curr Biol 17(9):783–788. doi:10.1016/j.cub.2007.03.067
Karmali MA, Mascarenhas M, Shen S, Ziebell K, Johnson S, Reid-Smith R, Isaac-Renton J, Clark C, Rahn K, Kaper JB (2003) Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J Clin Microbiol 41(11):4930–4940
Blasche S, Mortl M, Steuber H, Siszler G, Nisa S, Schwarz F, Lavrik I, Gronewold TM, Maskos K, Donnenberg MS, Ullmann D, Uetz P, Kogl M (2013) The E. coli effector protein NleF is a caspase inhibitor. PLoS ONE 8(3):e58937. doi:10.1371/journal.pone.0058937
Acknowledgments
We apologize to colleagues whose work could not be cited owing to space limitations and the defined focus of the review. Work in Dr. Shan Li’s laboratory is supported by the National Natural Science Foundation of China (NSFC) NO. 31470245 to S.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, J., Hu, J., Zhang, Y. et al. Hijacking of death receptor signaling by bacterial pathogen effectors. Apoptosis 20, 216–223 (2015). https://doi.org/10.1007/s10495-014-1068-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-1068-y