Abstract
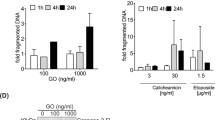
DNA fragmentation factor 40 (DFF40) is an endonuclease that acts downstream in the apoptotic cascade. The objective of this study was to generate a novel humanized chimeric protein with human DFF40 fused with GM-CSF for targeting acute myeloid leukemia (AML) cells. cDNA cloning of human DFF40 and GM-CSF was done and the chimeric gene GM-CSF-DFF40 was generated by overlap extension PCR. The fusion protein was expressed in E.coli, purified, refolded and characterized. In vitro cytotoxicity was evaluated on various AML cell lines. Treated cell lines were screened for various morphological and biochemical changes that are characteristic of apoptosis, by different assays like annexin V-FITC staining, TUNEL assay, JC-1 staining and immunocytochemistry of pro-apoptotic proteins and caspases. Cell cycle analysis of treated cells was done to quantify the percentage of apoptotic cells. The chimeric protein was found to be cytotoxic to AML cells in a dose and time dependent manner. Morphological changes such as formation of apoptotic bodies were revealed by microscopic examination of treated cells after staining. Immunocytochemical staining demonstrated biochemical changes such as changes in mitochondrial membrane potential, mitochondrial co-localization of Bax, cytochrome c release, presence of activated caspase-3 and DNA fragmentation. FACS analysis proved the presence of apoptotic cells following treatment. The chimeric protein GM-CSF-DFF40 was found to mediate targeted killing of AML cells by inducing apoptosis. Thus, this chimeric construct can act as a prospective candidate for targeted therapy of AML and other malignancies where GM-CSF receptor expression is upregulated.









Similar content being viewed by others
References
Burgess AW, Camakaris J, Metcalf D (1977) Purification and properties of colon stimulating factor from mouse lung-conditioned medium. J Biol Chem 252:1998–2003
Burgess AW, Metcalf D (1980) The nature and action of granulocyte-macrophage colony stimulating factors. Blood 56:947–958
Park LS, Waldron PE, Friend D (1989) Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood 74:56–65
Miyagawa K, Chiba S, Shibuya K (1990) Frequent expression of receptors for granulocyte-macrophage colony-stimulating factor on human nonhematopoietic tumor cell lines. J Cell Physiol 143:483–487
Perentesis JP, Waddick KG, Bendel AE (1997) Induction of apoptosis in multidrug- resistant and radiation-resistant acute myeloid leukemia cells by a recombinant fusion toxin directed against the human granulocyte macrophage colony-stimulating factor receptor. Clin Cancer Res 3:347–355
Kreitman RJ, Pastan I (1997) Recombinant toxins containing human granulocyte-macrophage colony-stimulating factor and either pseudomonas exotoxin or diphtheria toxin kill gastrointestinal cancer and leukemia cells. Blood 90:252–259
Lappi D, Martineau D, Sarmientos P (1993) Characterization of a saporin mitotoxin specifically cytotoxic to cells bearing the granulocyte-macrophage colony-stimulating factor receptor. Growth Factors 9:31–39
Frankel AE, Powell BL, Hall PD, Case LD, Kreitman RJ (2002) Phase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemia. Clin Cancer Res 8:1004–1013
Mathew M, Verma RS (2009) Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Sci 100:1359–1365
Stahnke B, Thepen T, Stocker M (2008) Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol Cancer Ther 7:2924–2932
Antignani A, Youle RJ (2005) A chimeric protein induces tumor cell apoptosis by delivering the human Bcl-2 family BH3-only protein Bad. Biochemistry 44:4074–4082
Zhang JH, Xu M (2000) DNA fragmentation in apoptosis. Cell Res 10:205–211
Liu X, Zou H, Widlak P, Garrard W, Wang X (1999) Activation of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease). J Biol Chem 274:13836–13840
Ben-Yehudah A, Aqeilan R, Robashkevich D, Lorberboum-Galski H (2003) Using apoptosis for targeted cancer therapy by a new gonadotropin releasing hormone-DNA fragmentation factor 40 chimeric protein. Clin Cancer Res 9:1179–1190
Tsumoto K, Umetsu M, Kumagai I (2004) Role of arginine in protein refolding, solubilization and purification. Biotechnol Prog 20:1301–1308
Kurata H, Arai T, Yokota T, Arai K (1995) Differential expression of granulocyte-macrophage colony-stimulating factor and IL-3 receptor subunits on human CD34 + cells and leukemic cell lines. J Allergy Clin Immunol 96:1083–1099
Heaney ML, Vera JC, Raines MA, Golde DW (1995) Membrane-associated and soluble granulocyte/macrophage-colony-stimulating factor receptor alpha subunits are independently regulated in HL-60 cells. Proc Natl Acad Sci USA 92:2365–2369
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45:528–537
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi X-G, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Wetzler M, Byrd JC, Bloomfield CD (2010) Acute and chronic myeloid leukemia. In: Longo D (ed) Harrison’s hematology and oncology. McGraw-Hill, New York, pp 166–181
Pulte D, Gondos A, Brenner H (2010) Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006–2010. Ann Oncol 21:335–341
MacFarlane M, Williams AC (2004) Apoptosis and disease: a life or death decision. EMBO Rep 5:674–678
Testa U, Riccioni R (2007) Deregulation of apoptosis in acute myeloid leukemia. Haematologica 92:81–94
Acknowledgments
This work was supported by Grant to RSV by the Department of Biotechnology, Govt. of India (BT/PR13463/PID/06/488/2010) and by fellowship to MM by the Indian Council of Medical Research (No. 3/1/3/JRF/37/MPD/2004(32403)).
Conflict of interest
The authors declare that there is no commercial or financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathew, M., Zaineb, K.C. & Verma, R.S. GM-CSF-DFF40: a novel humanized immunotoxin induces apoptosis in acute myeloid leukemia cells. Apoptosis 18, 882–895 (2013). https://doi.org/10.1007/s10495-013-0840-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0840-8