Abstract
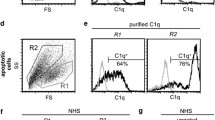
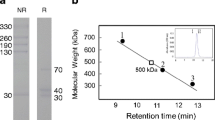
Apoptosis profoundly alters the carbohydrate layer coating the membrane of eukaryotic cells. Previously we showed that apoptotic cells became reactive with the α2,6-sialyl-specific lectin from Sambucus nigra agglutinin (SNA), regardless of their histological origin and the nature of the apoptotic stimulus. Here we reveal the basis of the phenomenon by showing that in apoptotic cancer cell lines SNA reactivity was mainly associated with a 67 kDa glycoprotein which we identified by MALDI-TOF/TOF and immunoblot analysis as bovine vitronectin (bVN). bVN was neither present in non-apoptotic cells, nor in cells induced to apoptosis in serum-free medium, indicating that its uptake from the cell culture serum occurred only during apoptosis. The bVN molecules associated with apoptotic cancer cell lines represented minor isoforms, lacking the carboxyterminal sequence and paradoxically containing a few α2,6-linked sialic acid residues. Despite their poor α2,6-sialylation, these bVN molecules were sufficient to turn apoptotic cells to SNA reactivity, which is a late apoptotic event occurring in cells positive to both annexin-V and propidium iodide. Unlike in cancer cell lines, the major bVN form taken up by apoptotic neutrophils and mononuclear cells was a 80 kDa form. In apoptotic SW948 cells we also detected the α2,6-sialylated forms of the stress-70 mitochondrial precursor (mortalin) and of tubulin-β2C. These data indicate that the acquisition of vitronectin isoforms from the environment is a general, although cell specific phenomenon, potentially playing an important role in post-apoptotic events and that the α2,6-sialylation of intracellular proteins is a new kind of posttranslational modification associated with apoptosis.







Similar content being viewed by others
Abbreviations
- An-V:
-
Annexin-V
- bVN:
-
Bovine vitronectin
- FCS:
-
Fetal calf serum
- IEF:
-
Isoelectrofocusing
- PARP:
-
Poly (ADP-ribose) polymerase
- PBMC:
-
Peripheral blood mononuclear cells
- PI:
-
Propidium iodide
- PMN:
-
Polymorphonuclear neutrophils
- PNGase F:
-
Peptide N-glycanase F
- SNA:
-
Sambucus nigra agglutinin
- TPEN:
-
N,N,N′,N′-tetrakis-(2-pyridylmethyl)ethylenediamine
References
Erwig LP, Henson PM (2008) Clearance of apoptotic cells by phagocytes. Cell Death Differ 15:243–250
Ravichandran KS, Lorenz U (2007) Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7:964–974
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Kinchen JM, Ravichandran KS (2007) Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci 120:2143–2149
Vandivier RW, Henson PM, Douglas IS (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129:1673–1682
Bilyy R, Kit Y, Hellman U, Tryndyak V, Kaminskyy V, Stoika R (2005) In vivo expression and characteristics of novel alpha-d-mannose-rich glycoprotein markers of apoptotic cells. Cell Biol Int 29:920–928
Bilyy R, Stoika R (2007) Search for novel cell surface markers of apoptotic cells. Autoimmunity 40:249–253
Franz S, Herrmann K, Fuhrnrohr B, Sheriff A, Frey B, Gaipl US, Voll RE, Kalden JR, Jack HM, Herrmann M (2007) After shrinkage apoptotic cells expose internal membrane-derived epitopes on their plasma membranes. Cell Death Differ 14:733–742
Hall SE, Savill JS, Henson PM, Haslett C (1994) Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J Immunol 153:3218–3227
Batisse C, Marquet J, Greffard A, Fleury-Feith J, Jaurand MC, Pilatte Y (2004) Lectin-based three-color flow cytometric approach for studying cell surface glycosylation changes that occur during apoptosis. Cytometry A 62:81–88
Brockhausen I, Lehotay M, Yang JM, Qin W, Young D, Lucien J, Coles J, Paulsen H (2002) Glycoprotein biosynthesis in porcine aortic endothelial cells and changes in the apoptotic cell population. Glycobiology 12:33–45
Franz S, Frey B, Sheriff A, Gaipl US, Beer A, Voll RE, Kalden JR, Herrmann M (2006) Lectins detect changes of the glycosylation status of plasma membrane constituents during late apoptosis. Cytometry A 69:230–239
Rapoport E, Le Pendu J (1999) Glycosylation alterations of cells in late phase apoptosis from colon carcinomas. Glycobiology 9:1337–1345
Rapoport EM, Sapot’ko YB, Pazynina GV, Bojenko VK, Bovin NV (2005) Sialoside-binding macrophage lectins in phagocytosis of apoptotic bodies. Biochemistry (Mosc) 70:330–338
Sarter K, Mierke C, Beer A, Frey B, Fuhrnrohr BG, Schulze C, Franz S (2007) Sweet clearance: involvement of cell surface glycans in the recognition of apoptotic cells. Autoimmunity 40:345–348
Malagolini N, Chiricolo M, Marini M, Dall’Olio F (2009) Exposure of α2,6-sialylated lactosaminic chains marks apoptotic and necrotic death in different cell types. Glycobiology 19:172–181
Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ (1987) The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α 2–6)Gal/GalNAc sequence. J Biol Chem 262:1596–1601
Weinstein J, de Souza-e-Silva U, Paulson JC (1982) Purification of a Gal β 1,4GlcNAc α 2,6 sialyltransferase and a Gal β 1,3(4)GlcNAc α 2,3 sialyltransferase to homogeneity from rat liver. J Biol Chem 257:13835–13844
Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC (1987) Primary structure of β-galactoside α 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem 262:17735–17743
Dall’Olio F (2000) The sialyl-α2,6-lactosaminyl-structure: biosynthesis and functional role. Glycoconj J 17:669–676
Deutsch HF (1954) Fetuin: the mucoprotein of fetal calf serum. J Biol Chem 208:669–678
Barnes DW, Silnutzer J (1983) Isolation of human serum spreading factor. J Biol Chem 258:12548–12552
Spik G, Bayard B, Fournet B, Strecker G, Bouquelet S, Montreuil J (1975) Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett 50:296–299
Chiricolo M, Malagolini N, Bonfiglioli S, Dall’Olio F (2006) Phenotypic changes induced by expression of β-galactoside α2,6 sialyltransferase I in the human colon cancer cell line SW948. Glycobiology 16:146–154
Dall’Olio F, Chiricolo M, Lollini P, Lau JT (1995) Human colon cancer cell lines permanently expressing α2,6-sialylated sugar chains by transfection with rat β-galactoside α 2,6 sialyltransferase cDNA. Biochem Biophys Res Commun 211:554–561
Preissner KT, Reuning U (2011) Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost 37:408–424
Kitagaki-Ogawa H, Yatohgo T, Izumi M, Hayashi M, Kashiwagi H, Matsumoto I, Seno N (1990) Diversities in animal vitronectins. Differences in molecular weight, immunoreactivity and carbohydrate chains. Biochim Biophys Acta 1033:49–56
Nakashima N, Miyazaki K, Ishikawa M, Yatohgo T, Ogawa H, Uchibori H, Matsumoto I, Seno N, Hayashi M (1992) Vitronectin diversity in evolution but uniformity in ligand binding and size of the core polypeptide. Biochim Biophys Acta 1120:1–10
Stepanek O, Brdicka T, Angelisova P, Horvath O, Spicka J, Stockbauer P, Man P, Horejsi V (2011) Interaction of late apoptotic and necrotic cells with vitronectin. PLoS ONE 6:e19243
Ogawa H, Yoneda A, Seno N, Hayashi M, Ishizuka I, Hase S, Matsumoto I (1995) Structures of the N-linked oligosaccharides on human plasma vitronectin. Eur J Biochem 230:994–1000
Stockmann A, Hess S, Declerck P, Timpl R, Preissner KT (1993) Multimeric vitronectin. Identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem 268:22874–22882
Sano K, Asanuma-Date K, Arisaka F, Hattori S, Ogawa H (2007) Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration. Glycobiology 17:784–794
Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL (2005) Hypersialylation of β1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res 65:4645–4652
Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL (2002) Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem 277:32830–32836
Sanchez-Ruderisch H, Detjen KM, Welzel M, Andre S, Fischer C, Gabius HJ, Rosewicz S (2011) Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1-integrin. Cell Death Differ 18:806–816
Savill J, Dransfield I, Hogg N, Haslett C (1990) Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343:170–173
Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM (1992) Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol 149:4029–4035
Fadok VA, Warner ML, Bratton DL, Henson PM (1998) CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J Immunol 161:6250–6257
Meesmann HM, Fehr EM, Kierschke S, Herrmann M, Bilyy R, Heyder P, Blank N, Krienke S, Lorenz HM, Schiller M (2010) Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci 123:3347–3356
Bilyy RO, Shkandina T, Tomin A, Munoz LE, Franz S, Antonyuk V, Kit YY, Zirngibl M, Furnrohr BG, Janko C, Lauber K, Schiller M, Schett G, Stoika RS, Herrmann M (2012) Macrophages discriminate glycosylation patterns of apoptotic cell-derived microparticles. J Biol Chem 287:496–503
Bae HB, Zmijewski JW, Deshane JS, Zhi D, Thompson LC, Peterson CB, Chaplin DD, Abraham E (2012) Vitronectin inhibits neutrophil apoptosis through activation of integrin-associated signaling pathways. Am J Respir Cell Mol Biol 46:790–796
Seiffert D (1997) Constitutive and regulated expression of vitronectin. Histol Histopathol 12:787–797
Deocaris CC, Kaul SC, Wadhwa R (2009) The versatile stress protein mortalin as a chaperone therapeutic agent. Protein Pept Lett 16:517–529
Garrido C, Solary E (2003) A role of HSPs in apoptosis through “protein triage”? Cell Death Differ 10:619–620
Creagh EM, Carmody RJ, Cotter TG (2000) Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res 257:58–66
Beere HM (2004) “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI (1995) Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem 270:1705–1710
Gasnier F, Ardail D, Lerme F, Simonot C, Vaganay E, Louisot P, Gateau-Roesch O (1994) Further characterization of mitochondrial outer membrane: evidence for the presence of two endogenous sialylated glycoproteins. J Biochem (Tokyo) 116:643–648
Avidan A, Perlmutter M, Tal S, Oraki O, Kapp T, Krelin Y, Elkabets M, Dotan S, Apte RN, Lichtenstein RG (2009) Differences in the sialylation patterns of membrane stress proteins in chemical carcinogen-induced tumors developed in BALB/c and IL-1alpha deficient mice. Glycoconj J 26:1181–1195
Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D (2002) Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem 277:33664–33669
Ireland CM, Pittman SM (1995) Tubulin alterations in taxol-induced apoptosis parallel those observed with other drugs. Biochem Pharmacol 49:1491–1499
Hino M, Kijima-Suda I, Nagai Y, Hosoya H (2003) Glycosylation of the α- and b-tubulin by sialyloligosaccharides. Zool Sci 20:709–715
Cassatella MA, Cappelli R, Della BV, Grzeskowiak M, Dusi S, Berton G (1988) Interferon-gamma activates human neutrophil oxygen metabolism and exocytosis. Immunology 63:499–506
Chai F, Truong-Tran AQ, Evdokiou A, Young GP, Zalewski PD (2000) Intracellular zinc depletion induces caspase activation and p21 Waf1/Cip1 cleavage in human epithelial cell lines. J Infect Dis 182(Suppl 1):S85–S92
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Acknowledgments
We thank Dr. M.C. Betts for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malagolini, N., Catera, M., Osorio, H. et al. Apoptotic cells selectively uptake minor glycoforms of vitronectin from serum. Apoptosis 18, 373–384 (2013). https://doi.org/10.1007/s10495-013-0812-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0812-z