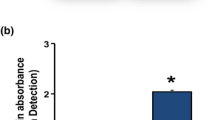
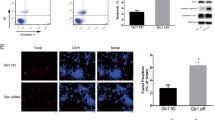
Abstract
Emerging evidence implicates novel roles for post-translational prenylation (i.e., farnesylation and geranylgeranylation) of various signaling proteins in a variety of cellular functions including hormone secretion, survival and apoptosis. In the context of cellular apoptosis, it has been shown previously that caspase-3 activation, a hallmark of mitochondrial dysregulation, promotes hydrolysis of several key cellular proteins. We report herein that exposure of insulin-secreting INS 832/13 cells or normal rat islets to etoposide leads to significant activation of caspase-3 and subsequent degradation of the common α-subunit of farnesyl/geranylgeranyl transferases (FTase/GGTase). Furthermore, the above stated signaling steps were prevented by Z-DEVD-FMK, a known inhibitor of caspase-3. In addition, treatment of cell lysates with recombinant caspase-3 also caused FTase/GGTase α-subunit degradation. Moreover, nifedipine, a calcium channel blocker, markedly attenuated etoposide-induced caspase-3 activation, FTase/GGTase α-subunit degradation in INS 832/13 cells and normal rat islets. Further, nifedipine significantly restored etoposide-induced loss in metabolic cell viability in INS 832/13 cells. Based on these findings, we conclude that etoposide induces loss in cell viability by inducing mitochondrial dysfunction, caspase-3 activation and degradation of FTase/GGTase α-subunit. Potential significance of these findings in the context of protein prenylation and β-cell survival are discussed.






Similar content being viewed by others
Abbreviations
- CSP3:
-
Caspase-3
- ET:
-
Etoposide
- FTase α:
-
Farnesyltransferase α subunit
- GGTase α:
-
Geranylgeranyltransferase α subunit
- Δ FTase/GGTase:
-
Hydrolytic product of FTase/GGTase α-subunit
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Nif:
-
Nifedipine
References
Kowluru A (2010) Small G proteins in islet beta-cell function. Endocr Rev 31:52–78
Robertson RP, Seaquist ER, Walseth TF (1991) G proteins and modulation of insulin secretion. Diabetes 40:1–6
Cox AD, Der CJ (1992) Protein prenylation: more than just glue? Curr Opin Cell Biol 4:1008–1016
Metz SA, Rabaglia ME, Stock JB, Kowluru A (1993) Modulation of insulin secretion from normal rat islets by inhibitors of the post-translational modifications of GTP-binding proteins. Biochem J. 295:31–40
Kowluru A (2003) Regulatory roles for small G proteins in the pancreatic beta-cell: lessons from models of impaired insulin secretion. Am J Physiol: Endocrinol Metab 285:E669–E684
Casey PJ, Seabra MC (1996) Protein prenyltransferases. J Biol Chem 271:5289–5292
Lane KT, Beese LS (2006) Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res 47:681–699
Amin R, Chen HQ, Tannous M, Gibbs R, Kowluru A (2002) Inhibition of glucose- and calcium-induced insulin secretion from betaTC3 cells by novel inhibitors of protein isoprenylation. J Pharmacol Exp Ther 303:82–88
Veluthakal R, Kaur H, Goalstone M, Kowluru A (2007) Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56:204–210
Kowluru A (2008) Protein prenylation in glucose-induced insulin secretion from the pancreatic islet beta-cell: a perspective. J Cell Mol Med 12:164–173
Kowluru A, Veluthakal R, Rhodes CJ, Kamath V, Syed I, Koch BJ (2010) Protein farnesylation-dependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells. Diabetes 59:967–977
Goalstone M, Kamath V, Kowluru A (2010) Glucose activates prenyltransferases in pancreatic islet beta-cells. Biochem Biophys Res Commun 391:895–898
Kim KW, Chung HH, Chung CW et al (2001) Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis. Oncogene 20:358–366
Collier JJ, Fueger PT, Hohmeier HE, Newgard CB (2006) Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55:1398–1406
Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529:57–68
Sharma AK, Rohrer B (2004) Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem 279:35564–35572
Tripathi A, Chaube SK (2012) High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17:439–448
Smith MA, Schnellmann RG (2012) Calpains mitochondria and apoptosis. Cardiovasc Res. doi:10.1093/cvr/cvs163
Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2000) Distinct pathways for stimulation of cytochrome c release by etoposide. J Biol Chem 275:32438–32443
Parihar A, Parihar MS, Ghafourifar P (2008) Significance of mitochondrial calcium and nitric oxide for apoptosis of human breast cancer cells induced by tamoxifen and etoposide. Int J Mol Med 21:317–324
Sorkin EM, Clissold SP, Brogden RN (1985) Nifedipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in ischaemic heart disease, hypertension and related cardiovascular disorders. Drugs. 30:182–274
Bean BP (1989) Classes of calcium channels in vertebrate cells. Annu Rev Physiol 51:367–384
Sane KM, Mynderse M, LaLonde DT et al (2010) A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J Pharmacol Exp Ther 333:23–33
Geryk-Hall M, Yang Y, Hughes DP (2010) Driven to death: inhibition of farnesylation increases ras activity in osteosarcoma and promotes growth arrest and cell death. Mol Cancer Ther 9:1111–1119
Li G, Segu VB, Rabaglia ME, Luo RH, Kowluru A, Metz SA (1998) Prolonged depletion of guanosine triphosphate induces death of insulin-secreting cells by apoptosis. Endocrinology 139:3752–3762
Wang Y, Gao L, Li Y, Chen H, Sun Z (2011) Nifedipine protects INS-1 beta-cell from high glucose-induced ER Stress and apoptosis. Int J Mol Sci 12:7569–7580
Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 61:848–856
Kyathanahalli CN, Kowluru A (2011) A farnesylated G-protein suppresses Akt phosphorylation in INS 832/13 cells and normal rat islets: regulation by pertussis toxin and PGE(2). Biochem Pharmacol 81:1237–1247
Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M (1992) Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci USA 89:3000–3004
Prokocimer M, Davidovich M, Nissim-Rafinia M et al (2009) Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med 13:1059–1085
Chang SY, Hudon-Miller SE, Yang SH et al (2012) Inhibitors of protein geranylgeranyltransferase-I lead to prelamin A accumulation in cells by inhibiting ZMPSTE24. J Lipid Res 53:1176–1182
Acknowledgments
This research was supported in part by a Merit Review award [to AK; 1BX000469] from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Biomedical Laboratory Research and Development], and by the NIH/NIDDK [RO1 DK74921]. AK is also the recipient of a Senior Research Career Scientist Award from the Department of VA. The authors would like to thank Prof. Chris Newgard for INS 832/13 cells. We thank Dr. Wasanthi Subasinghe for excellent technical assistance in these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arora, D.K., Mohammed, A.M. & Kowluru, A. Nifedipine prevents etoposide-induced caspase-3 activation, prenyl transferase degradation and loss in cell viability in pancreatic β-cells. Apoptosis 18, 1–8 (2013). https://doi.org/10.1007/s10495-012-0763-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0763-9