Abstract
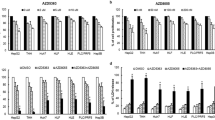
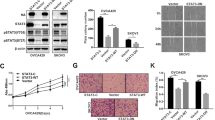
Recently, we reported that sMEK1 is down-regulated in cancer cells and tissues, and that it enhances the pro-proliferative effect as a novel pro-apoptotic protein. However, the biological mechanism of the sMEK1 tumor suppressor in the cellular signal pathway has not been well understood. In our current work, we examined whether sMEK1 could promote the cytotoxic activity of gemcitabine in the human ovarian carcinoma system. Initially, we attempted to use a treatment of gemcitabine traditional chemotherapeutic agent and over-expression of sMEK1 in OVCAR-3 cancer cells. The combined treatment of sMEK1 and gemcitabine was more effective at inhibiting cell proliferation than either chemotherapeutic agent treatment alone. In addition, sMEK1 actively contributes to cell migration through its ability to promote gemcitabine-inhibited cell migration in tumorigenesis. Cell cycle-related proteins are highly associated with the down-regulation of cyclin D1 and CDK4, and the promotion of p16 and p27 as a cyclin-dependent kinase inhibitor. At the same time, sMEK1 arrests cell cycle progression in the G1–G0 phase, and activates p53 and p21 expression, whereas Bcl-2 and Bcl-xL protein expression is reduced. Additionally, sMEK1 and gemcitabine suppresses the phosphorylation of signaling modulators downstream of PI3K, such as PDK1 and Akt. The p53 and p21 promoter luciferase activities were promoted by either sMEK1 or gemcitabine, and sMEK1 and gemcitabine combined additively activated the promoter further. Furthermore, as expected, sMEK1 plus gemcitabine markedly reduced the phosphorylation of p70S6K and the phosphorylation of 4E-BP1, which is one of the best characterized targets of the mTOR complex cascade. Taken together, these results provide evidence that sMEK1 can effectively regulate the pro-apoptotic activity of gemcitabine through the up-regulation of p53 expression.




Similar content being viewed by others
Abbreviations
- sMEK1:
-
Suppressor of MEK null 1
- PP4R3:
-
Protein phosphatase 4 regulatory subunit 3
- Gemcitabine:
-
2′deoxy-2′2′-difluorocytidine monohydrochloride
- MDR:
-
Multidrug resistance
- FITC:
-
Fluorescein isothiocyanate
- PI:
-
Propidium iodide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide
References
Gavin AC, Bosche M, Krause R et al (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141–147
Gingras AC, Caballero M, Zarske M et al (2005) A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity. Mol Cell Proteomics 4:1725–1740
Keogh MC, Kim JA, Downey M et al (2006) A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 439:497–501
Huang X, Cheng A, Honkanen RE (1997) Genomic organization of the human PP4 gene encoding a serine/threonine protein phosphatase (PP4) suggests a common ancestry with PP2A. Genomics 44:336–343
Andreeva AV, Kutuzov MA (2001) PPP family of protein Ser/Thr phosphatases: two distinct branches? Mol Biol Evol 18:448–452
Zhou G, Mihindukulasuriya KA, MacCorkle-Chosnek RA et al (2002) Protein phosphatase 4 is involved in tumor necrosis factor-alpha-induced activation of c-Jun N-terminal kinase. J Biol Chem 277:6391–6398
Cohen PT, Philp A, Vázquez-Martin C (2005) Protein phosphatase 4-from obscurity to vital functions. FEBS Lett 579:3278–3286
Zhang X, Ozawa Y, Lee H et al (2005) Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev 19:827–839
Chowdhury D, Xu X, Zhong X et al (2008) A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell 31:33–46
Mourtada-Maarabouni M, Williams GT (2008) Protein phosphatase 4 regulates apoptosis, proliferation and mutation rate of human cells. Biochim Biophys Acta 1783:1490–1502
Nakada S, Chen GI, Gingras AC et al (2008) PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep 9:1019–1026
Bertram PG, Choi JH, Carvalho J et al (2000) Tripartite regulation of Gln3p by TOR, Ure2p, and phosphatases. J Biol Chem 275:35727–35733
Mihindukulasuriya KA, Zhou G, Qin J et al (2004) Protein phosphatase 4 interacts with and down-regulates insulin receptor substrate 4 following tumor necrosis factor-alpha stimulation. J Biol Chem 279:46588–46594
Yoon YS, Lee MW, Ryu D et al (2010) Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc Natl Acad Sci U S A 107:17704–17709
Dong SM, Byun HJ, Kim BR et al (2012) Tumor suppressor BLU enhances pro-apoptotic activity of sMEK1 through physical interaction. Cell Signal 24:1208–1214
Plunkett W, Huang P, Searcy CE et al (1996) Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol 23:3–15
Peters GJ, Van Moorsel CJ, Lakerveld B et al (2006) Effects of gemcitabine on cis-platinum-DNA adduct formation and repair in a panel of gemcitabine and cisplatin-sensitive or -resistant human ovarian cancer cell lines. Int J Oncol 28:237–244
Toschi L, Finocchiaro G, Bartolini S et al (2005) Role of gemcitabine in cancer therapy. Future Oncol 1:7–17
Bhoola S, Hoskins WJ (2006) Diagnosis and management of epithelial ovarian cancer. Obstet Gynecol 107:1399–1410
Trock BJ, Leonessa F, Clarke R (1997) Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst 89:917–931
Leith C (1998) Multidrug resistance in leukemia. Curr Opin Hematol 5:287–291
Szakacs G, Jakab K, Antal F et al (1998) Diagnostics of multidrug resistance in cancer. Pathol Oncol Res 4:251–257
Van Nimwegen MJ, Van Dewater B (2007) Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol 73:597–609
Morgan L, Nicholson RI, Hiscox S (2008) SRC as a therapeutic target in breast cancer. Endocr Metab Immune Disord Drug Targets 8:273–278
Schlessinger J (2000) New roles for Src kinases in control of cell survival and angiogenesis. Cell 100:293–296
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Antonsson B, Martinou JC (2000) The Bcl-2 protein family. Exp Cell Res 256:50–57
Yin XM, Oltvai ZN, Korsmeyer SJ (1994) BH1 and BH2 domain of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369:321–323
Reed JC (1997) Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 34:9–19
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153–164
Malaguarnera L (2004) Implications of apoptosis regulators in tumorigenesis. Cancer Metastasis Rev 23:367–387
Lorusso D, Di Stefano A, Fanfani F et al (2006) Role of gemcitabine in ovarian cancer treatment. Ann Oncol 17:v188–v194
Bergman AM, Ruiz van Haperen VW, Veerman G et al (1996) Synergistic interaction between Cisplatin and Gemcitabine in vitro. Clin Cancer Res 2:521–530
Wang S, Zhang H, Cheng L et al (2010) Analysis of the cytotoxic activity of carboplatin and gemcitabine combination. Anticancer Res 30:4573–4578
Yu WD, Ma Y, Flynn G et al (2010) Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 9:3022–3029
Acknowledgments
This work was supported by a grant from the National Cancer Center, Korea (NCC-1210470-1). We thank Dr. S.A. Martinis (Department of Biochemistry, University of Illinois at Urbana-Champaign, IL, USA) for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Byun, HJ., Kim, BR., Yoo, R. et al. sMEK1 enhances gemcitabine anti-cancer activity through inhibition of phosphorylation of Akt/mTOR. Apoptosis 17, 1095–1103 (2012). https://doi.org/10.1007/s10495-012-0751-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0751-0