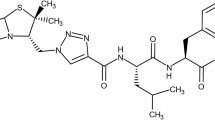
Abstract
d-Penicillamine (3,3-dimethyl-d-cysteine; DP) is an FDA-approved redox-active d-cysteine-derivative with antioxidant, disulfide-reducing, and metal chelating properties used therapeutically for the control of copper-related pathology in Wilson’s disease and reductive cystine-solubilization in cystinuria. Based on the established sensitivity of metastatic melanoma cells to pharmacological modulation of cellular oxidative stress, we tested feasibility of using DP for chemotherapeutic intervention targeting human A375 melanoma cells in vitro and in vivo. DP treatment induced caspase-dependent cell death in cultured human metastatic melanoma cells (A375, G361) without compromising viability of primary epidermal melanocytes, an effect not observed with the thiol-antioxidants N-acetyl-l-cysteine (NAC) and dithiothreitol. Focused gene expression array analysis followed by immunoblot detection revealed that DP rapidly activates the cytotoxic unfolded protein response (UPR; involving phospho-PERK, phospho-eIF2α, Grp78, CHOP, and Hsp70) and the mitochondrial pathway of apoptosis with p53 upregulation and modulation of Bcl-2 family members (involving Noxa, Mcl-1, and Bcl-2). DP (but not NAC) induced oxidative stress with early impairment of glutathione homeostasis and mitochondrial transmembrane potential. SiRNA-based antagonism of PMAIP1 expression blocked DP-induced upregulation of the proapoptotic BH3-only effector Noxa and prevented downregulation of the Noxa-antagonist Mcl-1, rescuing melanoma cells from DP-induced apoptosis. Intraperitoneal administration of DP displayed significant antimelanoma activity in a murine A375 xenograft model. It remains to be seen if melanoma cell-directed induction of UPR and apoptosis using DP or improved DP-derivatives can be harnessed for future chemotherapeutic intervention.






Similar content being viewed by others
References
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16(1):5–24
Aplin AE, Kaplan FM, Shao Y (2011) Mechanisms of resistance to RAF inhibitors in melanoma. J Invest Dermatol 131(9):1817–1820
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS et al (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366(8):707–714
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107(12):4907–4916
Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A (2009) Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol 625(1–3):234–246
Wondrak GT (2009) Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal 11(12):3013–3069
Tew KD, Townsend DM (2011) Redox platforms in cancer drug discovery and development. Curr Opin Chem Biol 15(1):156–161
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591
Qiao S, Lamore SD, Cabello CM, Lesson JL, Munoz-Rodriguez JL, Wondrak GT (2012) Thiostrepton is an inducer of oxidative and proteotoxic stress that impairs viability of human melanoma cells but not primary melanocytes. Biochem Pharmacol 83(9):1229–1240
De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF et al (2011) Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell 20(3):400–413
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136(5):823–837
Wondrak GT (2007) NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radic Biol Med 43(2):178–190
Cabello CM, Bair WB III, Bause AS, Wondrak GT (2009) Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1. Biochem Pharmacol 78(4):344–354
Cabello CM, Bair WB III, Lamore SD, Ley S, Bause AS, Azimian S et al (2009) The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med 46(2):220–231
Cabello CM, Lamore SD, Bair WB 3rd, Qiao S, Azimian S, Lesson JL et al (2011) The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest New Drugs 30(4):1289–1301
Levy RS, Fisher M, Alter JN (1983) Penicillamine: review and cutaneous manifestations. J Am Acad Dermatol 8:548–558
Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (2007) Wilson’s disease. Lancet 369(9559):397–408
Wondrak GT, Cervantes-Laurean D, Roberts MJ, Qasem JG, Kim M, Jacobson EL et al (2002) Identification of alpha-dicarbonyl scavengers for cellular protection against carbonyl stress. Biochem Pharmacol 63(3):361–373
Lengfelder E, Elstner EF (1978) Determination of the superoxide dismutating activity of d-penicillamine copper. Hoppe Seylers Z Physiol Chem 359(6):751–757
Cohen JF, Elberling JA, DeMaster EG, Lin RC, Nagasawa HT (2000) N-terminal dipeptides of d(-)-penicillamine as sequestration agents for acetaldehyde. J Med Chem 43:1029–1033
Gupte A, Mumper RJ (2007) An investigation into copper catalyzed d-penicillamine oxidation and subsequent hydrogen peroxide generation. J Inorg Biochem 101(4):594–602
Havre PA, O’Reilly S, McCormick JJ, Brash DE (2002) Transformed and tumor-derived human cells exhibit preferential sensitivity to the thiol antioxidants, N-acetyl cysteine and penicillamine. Cancer Res 62(5):1443–1449
Wondrak GT, Jacobson MK, Jacobson EL (2006) Antimelanoma activity of apoptogenic carbonyl scavengers. J Pharmacol Exp Ther 316(2):805–814
Gupte A, Mumper RJ (2007) Copper chelation by d-penicillamine generates reactive oxygen species that are cytotoxic to human leukemia and breast cancer cells. Free Radic Biol Med 43(9):1271–1278
Cabello CM, Bair WB III, Ley S, Lamore SD, Azimian S, Wondrak GT (2009) The experimental chemotherapeutic N(6)-furfuryladenosine (kinetin-riboside) induces rapid ATP depletion, genotoxic stress, and CDKN1A (p21) upregulation in human cancer cell lines. Biochem Pharmacol 77(7):1125–1138
Lamore SD, Cabello CM, Wondrak GT (2010) The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperones 15(3):309–322
Cabello CM, Lamore SD, Bair WB, Davis AL, Azimian SM, Wondrak GT (2011) DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma. Free Radic Res 45(3):276–292
Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC (2010) Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem 285(43):33165–33174
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND et al (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129(7):1337–1349
Eriksson O, Fontaine E, Petronilli V, Bernardi P (1997) Inhibition of the mitochondrial cyclosporin A-sensitive permeability transition pore by the arginine reagent phenylglyoxal. FEBS Lett 409(3):361–364
Keuling AM, Andrew SE, Tron VA (2010) Inhibition of p38 MAPK enhances ABT-737-induced cell death in melanoma cell lines: novel regulation of PUMA. Pigment Cell Melanoma Res 23(3):430–440
Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V et al (2005) Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res 65(14):6282–6293
Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW Jr et al (2005) Differential regulation of Noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res 65(14):6294–6304
Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ (2006) Enhanced killing of melanoma cells by simultaneously targeting Mcl-1 and NOXA. Cancer Res 66(19):9636–9645
Weber A, Kirejczyk Z, Potthoff S, Ploner C, Hacker G (2009) Endogenous Noxa determines the strong proapoptotic synergism of the BH3-Mimetic ABT-737 with chemotherapeutic agents in human melanoma cells. Transl Oncol 2(2):73–83
Yu KS, Lee Y, Kim CM, Park EC, Choi J, Lim DS et al (2010) The protease inhibitor, elafin, induces p53-dependent apoptosis in human melanoma cells. Int J Cancer 127(6):1308–1320
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21(4):1249–1259
Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, deBock CE, Thorne RF et al (2008) Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res 68(16):6708–6717
Xu W, Trepel J, Neckers L (2011) Ras, ROS and proteotoxic stress: a delicate balance. Cancer Cell 20(3):281–282
Li J, Lee B, Lee AS (2006) Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 281(11):7260–7270
Guan D, Xu Y, Yang M, Wang H, Wang X, Shen Z (2010) N-acetyl cysteine and penicillamine induce apoptosis via the ER stress response-signaling pathway. Mol Carcinog 49(1):68–74
Jeitner TM, Lawrence DA (2001) Mechanisms for the cytotoxicity of cysteamine. Toxicol Sci 63(1):57–64
DeBerardinis RJ, Coughlin CR 2nd, Kaplan P (2008) Penicillamine therapy for pediatric cystinuria: experience from a cohort of American children. J Urol 180(6):2620–2623
Perrett D (1981) The metabolism and pharmacology of D-penicillamine in man. J Rheumatol Suppl 7:41–50
Winterbourn CC, Metodiewa D (1999) Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 27(3–4):322–328
Kim HR, Lee GH, Cho EY, Chae SW, Ahn T, Chae HJ (2009) Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci 122(Pt 8):1126–1133
Suh JK, Poulsen LL, Ziegler DM, Robertus JD (1999) Yeast flavin-containing monooxygenase generates oxidizing equivalents that control protein folding in the endoplasmic reticulum. Proc Natl Acad Sci USA 96(6):2687–2691
Krueger SK, Williams DE (2005) Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther 106(3):357–387
Poulsen LL, Ziegler DM (1977) Microsomal mixed-function oxidase-dependent renaturation of reduced ribonuclease. Arch Biochem Biophys 183(2):563–570
Suh JK, Robertus JD (2000) Yeast flavin-containing monooxygenase is induced by the unfolded protein response. Proc Natl Acad Sci USA 97(1):121–126
Chvapil M, Dorr R (2005) Single intratumoral injection of long-acting benzyl ester of d-penicillamine inhibits the growth of melanoma tumor in mice. Anticancer Drugs 16(7):757–762
de Groot-Besseling RR, Ruers TJ, Lamers-Elemans IL, Maass CN, de Waal RM, Westphal JR (2006) Angiostatin generating capacity and anti-tumour effects of d-penicillamine and plasminogen activators. BMC Cancer 6:149
Brem S, Grossman SA, Carson KA, New P, Phuphanich S, Alavi JB et al (2005) Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol 7(3):246–253
Wadhwa S, Mumper RJ (2010) Intracellular delivery of the reactive oxygen species generating agent d-penicillamine upon conjugation to poly-l-glutamic acid. Mol Pharm 7(3):854–862
Acknowledgments
Supported in part by Grants from the National Institutes of Health [R01CA122484, R03CA167580, ES007091, ES006694, Arizona Cancer Center Support Grant CA023074].
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, S., Cabello, C.M., Lamore, S.D. et al. d-Penicillamine targets metastatic melanoma cells with induction of the unfolded protein response (UPR) and Noxa (PMAIP1)-dependent mitochondrial apoptosis. Apoptosis 17, 1079–1094 (2012). https://doi.org/10.1007/s10495-012-0746-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0746-x