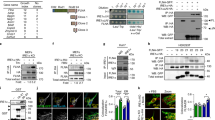
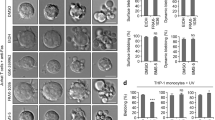
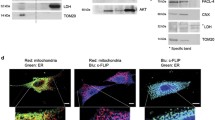
Abstract
FasR stimulation by Fas ligand leads to rapid formation of FasR microaggregates, which become signaling protein oligomerization transduction structures (SPOTS), through interactions with actin and ezrin, a structural step that triggers death-inducing signaling complex formation, in association with procaspase-8 activation. In some cells, designated as type I, caspase 8 directly activates effector caspases, whereas in others, known as type II, the caspase-mediated death signaling is amplified through mitochondria. Keratins are the intermediate filament (IF) proteins of epithelial cells, expressed as pairs in a lineage/differentiation manner. Hepatocyte IFs are made solely of keratins 8/18 (K8/K18), the hallmark of all simple epithelia. We have shown recently that in comparison to type II wild-type (WT) mouse hepatocytes, the absence of K8/K18 IFs in K8-null hepatocytes leads to more efficient FasR-mediated apoptosis, in link with a type II/type I-like switch in FasR-death signaling. Here, we demonstrate that the apoptotic process occurring in type I-like K8-null hepatocytes is associated with accelerated SPOTS elaboration at surface membrane, along with manifestation of FasR cap formation and internalization. In addition, the lipid raft organization is altered in K8-null hepatocytes. While lipid raft inhibition impairs SPOTS formation in both WT and K8-null hepatocytes, the absence of K8/K18 IFs in the latter sensitizes SPOTS to actin de-polymerization, and perturbs ezrin compartmentalization. Overall, the results indicate that the K8/K18 IF loss in hepatocytes alters the initial FasR activation steps through perturbation of ezrin/actin interplay and lipid raft organization, which leads to a type II/type I switch in FasR-death signaling.










Similar content being viewed by others
References
Kumar S (1999) Mechanisms mediating caspase activation in cell death. Cell Death Differ 6(11):1060–1066
Zheng TS, Flavell RA (1999) Apoptosis. All’s well that ends dead. Nature 400(6743):410–411
Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, Straus SE, Lenardo MJ (2004) SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol 167(4):735–744
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10(1):26–35
Barnhart BC, Alappat EC, Peter ME (2003) The CD95 type I/type II model. Semin Immunol 15(3):185–193
Park SM, Peter ME (2008) microRNAs and death receptors. Cytokine Growth Factor Rev 19(3–4):303–311
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15(22):2922–2933
Schutze S, Tchikov V, Schneider-Brachert W (2008) Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol 9(8):655–662
Hansen CG, Nichols BJ (2009) Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci 122(11):1713–1721
Kenworthy AK (2008) Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep 9(6):531–535
Lajoie P, Goetz JG, Dennis JW, Nabi IR (2009) Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol 185(3):381–385
Browman DT, Hoegg MB, Robbins SM (2007) The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol 17(8):394–402
Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW (2006) New consensus nomenclature for mammalian keratins. J Cell Biol 174(2):169–174
Coulombe PA, Wong P (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 6(8):699–706
Lane EB, Hogan BL, Kurkinen M, Garrels JI (1983) Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 303(5919):701–704
Lu H, Hesse M, Peters B, Magin TM (2005) Type II keratins precede type I keratins during early embryonic development. Eur J Cell Biol 84(8):709–718
Marceau N, Loranger A, Gilbert S, Daigle N, Champetier S (2001) Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem Cell Biol 79(5):543–555
Omary MB, Ku NO, Toivola DM (2002) Keratins: guardians of the liver. Hepatology 35(2):251–257
Oshima RG, Baribault H, Caulin C (1996) Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev 15(4):445–471
Ku NO, Soetikno RM, Omary MB (2003) Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology 37(5):1006–1014
Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB (2007) Keratins let liver live: mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology 46(5):1639–1649
Marceau N, Schutte B, Gilbert S, Loranger A, Henfling ME, Broers JL, Mathew J, Ramaekers FC (2007) Dual roles of intermediate filaments in apoptosis. Exp Cell Res 313(10):2265–2281
Oshima RG (2002) Apoptosis and keratin intermediate filaments. Cell Death Differ 9(5):486–492
Nagata S (1999) Fas ligand-induced apoptosis. Annu Rev Genet 33:29–55
Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A (1996) Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 2(1):80–86
de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C, Kahn A, Mignon A (1999) Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol 277(3 Pt 1):G702–G708
Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400(6747):886–891
Gilbert S, Loranger A, Daigle N, Marceau N (2001) Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol 154(4):763–773
Gilbert S, Loranger A, Marceau N (2004) Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol Cell Biol 24(16):7072–7081
Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L). Proc Natl Acad Sci USA 96(26):14871–14876
Gilbert S, Ruel A, Loranger A, Marceau N (2008) Switch in Fas-activated death signaling pathway as result of keratin 8/18-intermediate filament loss. Apoptosis 13(12):1479–1493. doi:10.1007/s10495-008-0274-x
Marceau N, Gilbert S, Loranger A (2004) Uncovering the roles of intermediate filaments in apoptosis. Methods Cell Biol 78:95–129
Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P (2009) Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4(11):1582–1590. doi:10.1038/nprot.2009.151
Zeidan YH, Jenkins RW, Hannun YA (2008) Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol 181(2):335–350. doi:10.1083/jcb.200705060
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Kabouridis PS, Janzen J, Magee AL, Ley SC (2000) Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol 30(3):954–963. doi:10.1002/1521-4141(200003)30:3<954:AID-IMMU954>3.0.CO;2-Y
Ismair MG, Hausler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B (2009) ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology 49(5):1673–1682
Bordeleau F, Galarneau L, Gilbert S, Loranger A, Marceau N (2010) Keratin 8/18 modulation of protein kinase C-mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol Biol Cell 21(10):1698–1713. doi:10.1091/mbc.E09-05-0373
Mathew J, Galarneau L, Loranger A, Gilbert S, Marceau N (2008) Keratin-protein kinase C interaction in reactive oxygen species-induced hepatic cell death through mitochondrial signaling. Free Radic Biol Med 45(4):413–424
Fais S, De Milito A, Lozupone F (2005) The role of FAS to ezrin association in FAS-mediated apoptosis. Apoptosis 10(5):941–947. doi:10.1007/s10495-005-0478-2
Giammarioli AM, Garofalo T, Sorice M, Misasi R, Gambardella L, Gradini R, Fais S, Pavan A, Malorni W (2001) GD3 glycosphingolipid contributes to Fas-mediated apoptosis via association with ezrin cytoskeletal protein. FEBS Lett 506(1):45–50
Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL (2006) Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 131(3):878–884. doi:10.1053/j.gastro.2006.06.013
Chaigne-Delalande B, Moreau JF, Legembre P (2008) Rewinding the DISC. Arch Immunol Ther Exp (Warsz) 56(1):9–14
Algeciras-Schimnich A, Pietras EM, Barnhart BC, Legembre P, Vijayan S, Holbeck SL, Peter ME (2003) Two CD95 tumor classes with different sensitivities to antitumor drugs. Proc Natl Acad Sci USA 100(20):11445–11450. doi:10.1073/pnas.2034995100
Haouzi D, Baghdiguian S, Granier G, Travo P, Mangeat P, Hibner U (2005) Three-dimensional polarization sensitizes hepatocytes to Fas/CD95 apoptotic signalling. J Cell Sci 118(Pt 12):2763–2773. doi:10.1242/jcs.02403
McNiven MA, Wolkoff AW, Hubbard A (2009) A stimulus needed for the study of membrane traffic in hepatocytes. Hepatology 50(2):345–348
Mazzone A, Tietz P, Jefferson J, Pagano R, LaRusso NF (2006) Isolation and characterization of lipid microdomains from apical and basolateral plasma membranes of rat hepatocytes. Hepatology 43(2):287–296
Tietz P, Jefferson J, Pagano R, Larusso NF (2005) Membrane microdomains in hepatocytes: potential target areas for proteins involved in canalicular bile secretion. J Lipid Res 46(7):1426–1432. doi:10.1194/jlr.M400412-JLR200
Moreno M, Molina H, Amigo L, Zanlungo S, Arrese M, Rigotti A, Miquel JF (2003) Hepatic overexpression of caveolins increases bile salt secretion in mice. Hepatology 38(6):1477–1488
Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287. doi:10.1038/nrm2866
Rossin A, Kral R, Lounnas N, Chakrabandhu K, Mailfert S, Marguet D, Hueber AO (2010) Identification of a lysine-rich region of Fas as a raft nanodomain targeting signal necessary for Fas-mediated cell death. Exp Cell Res 316(9):1513–1522. doi:10.1016/j.yexcr.2010.03.002
Algeciras-Schimnich A, Peter ME (2003) Actin dependent CD95 internalization is specific for Type I cells. FEBS Lett 546(2–3):185–188
Wald FA, Oriolo AS, Casanova ML, Salas PJ (2005) Intermediate filaments interact with dormant ezrin in intestinal epithelial cells. Mol Biol Cell 16(9):4096–4107. doi:10.1091/mbc.E05-03-0242
Ku NO, Omary MB (2006) A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol 174(1):115–125
Acknowledgments
We thank H. Baribault for the original WT and K8-null mouse colonies, R. Kemler for the TROMA-1 hybridoma, J. Lippincott-Schwartz for the PAGFP vector and A. Helenius for the caveolin-1-mRFP construct. This work was supported by the Canadian Institutes of Health Research (MOP 15529 to NM; MOP 49450 to JNL).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2012_733_MOESM1_ESM.tif
Online Resource 1: FasR capping is increased in absence of K8/K18 IFs. a Confocal imaging of FasR after the addition of FasL (100 ng/ml) for 60 min, showing more pronounced cap structure (white arrow) formation in K8-null than WT hepatocytes. b Quantification of the percentage of cells with caps at their surface as function of time following a stimulation with FasL (100 ng/ml) (TIFF 29865 kb)
10495_2012_733_MOESM2_ESM.tif
Online Resource 2: FasR internalization is observed only in cell without K8/K18 IFs. Confocal images of Jo2-PE at cell middle plan of WT and K8-null hepatocytes in presence of Jo2-PE (0.5 μg/ml) + PA (0.1 μg/ml), showing FasR internalization (red arrow in zoom panel) only in K8-null hepatocytes, starting at 30 min after being put back at 37 °C (TIFF 6852 kb)
10495_2012_733_MOESM3_ESM.tif
Online Resource 3: Faster FasR SPOTS formation in K8-null hepatocytes monitored by real-time confocal microscopy and alignment with caveolin-1. Real-time confocal imaging at the surface plan of WT (a) and K8-null (b) hepatocytes transfected with FasR-EGFP (green) and caveolin-1-mRFP (red), and stimulated with Jo2 (0.5 μg/ml) + PA (0.1 μg/ml), showing a faster FasR SPOTS formation (yellow circle) in K8-null compare to WT hepatocytes, and an alignment of caveolin-1 with FasR SPOTS. Images were taken each 30 s for a 30 min period (TIFF 11532 kb)
10495_2012_733_MOESM4_ESM.tif
Online Resource 4: Radixin co-distibrutes with actin and flotillin-2 at bile canaliculi but not with FasR. a Confocal imaging of radixin (green), actin (red) and nucleus (blue) in WT and K8-null hepatocytes, showing a co-distribution of actin and radixin at bile canaliculi of both cell types. b Confocal imaging of radixin (red), flotillin-2 (green) and nucleus (blue) in WT and K8-null hepatocytes, showing a co-distribution of flotillin-2 and radixin at bile canaliculi of both cell types; FasR stimulation led to the appearance of flotillin-2 at the surface membrane in K8-null hepatocytes, in co-localization with radixin. c Confocal imaging of radixin (green), FasR (red) and nucleus (blue) in WT and K8-null hepatocytes, revealing an absence of FasR and radixin co-localization (TIFF 21699 kb)
Rights and permissions
About this article
Cite this article
Gilbert, S., Loranger, A., Lavoie, J.N. et al. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis 17, 880–894 (2012). https://doi.org/10.1007/s10495-012-0733-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0733-2