Abstract
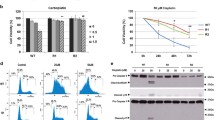
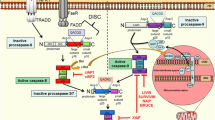
Resistance to cisplatin chemotherapy remains a major hurdle preventing effective treatment of many solid cancers. BAX and BAK are pivotal regulators of the mitochondrial apoptosis pathway, however little is known regarding their regulation in cisplatin resistant cells. Cisplatin induces DNA damage in both sensitive and resistant cells, however the latter exhibits a failure to initiate N-terminal exposure of mitochondrial BAK or mitochondrial SMAC release. Both phenotypes are highly sensitive to mitochondrial permeabilisation induced by exogenous BH3 domain peptides derived from BID, BIM, NOXA (which targets MCL-1 and A1), and there is no significant change in their prosurvival BCL2 protein expression profiles. Obatoclax, a small molecule inhibitor of pro-survival BCL-2 family proteins including MCL-1, decreases cell viability irrespective of platinum resistance status across a panel of cell lines selected for oxaliplatin resistance. In summary, selection for platinum resistance is associated with a block of mitochondrial death signalling upstream of BAX/BAK activation. Conservation of sensitivity to BH3 domain induced apoptosis can be exploited by agents such as obatoclax, which directly target the mitochondria and BCL-2 family.





Similar content being viewed by others
References
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108(2):153–164
Hanahan D (2000) Weinberg. RA. The hallmarks of cancer. Cell 100(1):57–70
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489
Kluck RM, Bossy-Wetzel E, Green DR (1997) Newmeyer DD The release of cytochrome c from mitochondria: a primary site for BCL-2 regulation of apoptosis. Science 275(5303):1132–1136
Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR et al (2002) The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J Biol Chem 277(1):439–444
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY et al (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410(6828):549–554
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102(1):33–42
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292(5517):727–730
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ et al (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8(12):1348–1358
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al (2005) Differential targeting of prosurvival BCL-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17(3):393–403
Letai A, Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ et al (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis serving as prototype cancer therapeutics. Cancer Cell 2(3):183–192
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17(4):525–535
Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P (2004) The first alpha helix of BAX plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell 16(5):807–818
Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K et al (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434(7035):864–870
Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S et al (2002) Calpain-mediated bid cleavage and calpain-independent BAK modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22(9):3003–3013
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS (2002) BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849
Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L et al (2005) Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 122(4):593–603
Cullen KJ, Yang Z, Schumaker L, Guo Z (2007) Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J Bioenerg Biomembr 39(1):43–50
Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ (2003) AKT2 inhibition of cisplatin-induced JNK/p38 and BAX activation by phosphorylation of ASK1 implication of AKT2 in chemoresistance*. J Biol Chem 278(26):23432–23440
Andrews PA, Albright KD (1992) Mitochondrial defects in cis-diamminedichloroplatinum(II)-resistant human ovarian carcinoma cells. Cancer Res 52(7):1895–1901
Ara G, Kusumoto T, Korbut TT, Cullere-Luengo F, Teicher BA (1994) cis-Diamminedichloroplatinum(II) resistant human tumor cell lines are collaterally sensitive to PtCl4(Rh-123)2: evidence for mitochondrial involvement. Cancer Res 54(6):1497–1502
Henkels KM, Turchi JJ (1999) Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines. Cancer Res 59(13):3077–3083
Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P et al (1998) Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396(6712):699–703
Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L et al (2004) Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 5(2):163–175
Pérez-Galán P, Roué G, Villamor N, Campo E, Colomer D (2007) The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing NOXA-mediated activation of BAK. Blood 109(10):4441–4449
Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR et al (2007) Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA 104(49):19512–19517
Ruffolo SC, Shore GC (2003) BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem 278(27):25039–25045
Wang GQ, Gastman BR, Wieckowski E, Goldstein LA, Gambotto A, Kim TH et al (2001) A role for mitochondrial BAK in apoptotic response to anticancer drugs. J Biol Chem 276(36):34307–34317
Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA et al (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9(5):351–365
Oltersdorf T et al (2005) An inhibitor of BCL-2 family proteins induces regression of solid tumours. Nature 435(7042):677–681
Balakrishnan K, Burger JA, Wierda WG, Gandhi V (2009) AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated MCL-1 induction and drug resistance. Blood 113(1):149–153
Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL et al (2010) Phase I dose finding studies of obatoclax (GX15-070), a small molecule Pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res 16(15):4038–4045
Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR (2000) BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer 82(2):436–440
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J et al (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463(7283):899–905
Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ et al (2009) Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ 16(7):1030–1039
Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK et al (2010) Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest 120(4):1310–1323
Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z et al (2008) Mechanisms of antileukemic activity of the novel BCL-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res 68(9):3413–3420
Smoot RL, Blechacz BR, Werneburg NW, Bronk SF, Sinicrope FA et al (2010) A BAX-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells. Cancer Res 70(5):1960–1969
Acknowledgements
This work was funded by Cancer Research UK and Action Cancer, and the Department for Education and Learning Northern Ireland. D.A.F. is a recipient of a Cancer Research UK Clinician Scientist Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nyree Crawford and Alex D. Chacko contributed equally to this article.
Rights and permissions
About this article
Cite this article
Crawford, N., Chacko, A.D., Savage, K.I. et al. Platinum resistant cancer cells conserve sensitivity to BH3 domains and obatoclax induced mitochondrial apoptosis. Apoptosis 16, 311–320 (2011). https://doi.org/10.1007/s10495-010-0561-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0561-1