Abstract
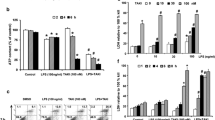
Nitric oxide (NO) is a reactive secondary mediator, which has been found to participate in cell cycle regulation and apoptosis in myeloid macrophages, the key effectors of inflammatory and innate immune responses. However, the molecular mechanisms of nitric oxide-induced death of myeloid macrophages are not well understood. In this study we have found that NO derived from S-nitrosoglutathione (GSNO) activates ASK1 in THP-1 human myeloid macrophages in a concentration and time-dependent manner. It also induces accumulation of HIF-1α protein in a concentration-dependent manner, which peaks at 4 h of exposure to 1 mM GSNO. GSNO does not affect the level of HIF-1α mRNA as detected by the RT-PCR. In addition, GSNO was found to induce accumulation of p53 in normal but not HIF-1α knockdown THP-1 cells, where expression of this protein was silenced by specific siRNA. It has also been found that GSNO-mediated accumulation of p53 depends on activation of ASK1 since no GSNO-induced p53 stabilisation was observed in THP-1 cells transfected with dominant-negative form of this kinase. However, in both HIF-1α knockdown THP-1 cells and those transfected with the dominant-negative form of ASK1, GSNO was able to induce cell death as detected by the MTS cell viability assay leading to an increase in release of LDH.





Similar content being viewed by others
References
Sumbayev VV, Yasinska IM (2007) Mechanisms of hypoxic signal transduction regulated by reactive nitrogen species. Scand J Immunol 65:399–406
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Sumbayev VV (2003) S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1 Arch. Biochem Biophys 415:133–136
Yasinska IM, Kozhukhar AV, Sumbayev VV (2004) S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch Biochem Biophys 428:198–203
Kyriakis JM (2000) Mammalian MAP kinase pathways. In Woodgett J (ed) Protein kinase functions. Oxford University Press, Oxford, pp 40–156
Finkel T (2000) Redox-dependent signal transduction. FEBS Lett 476:52–54
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606
Sumbayev VV, Yasinska IM (2005) Regulation of MAP kinase-dependent apoptotic pathway: implication of reactive oxygen and nitrogen species. Arch Biochem Biophys 436:406–412
Fuchs SY, Adler V, Pincus MR, Ronai Z (1998) MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA 95:10541–10546
Fuchs SY, Adler V, Buschmann T, Yin Z, Wu X, Jones SN, Ronai Z (1998) JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev 12:2658–2663
Achison M, Hupp TR (2003) Hypoxia attenuates the p53 response to cellular damage. Oncogene 22:3431–3440
Chen D, Li M, Luo J, Gu W (2003) Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem 278:13595–13598
Semenza GL (2002) HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8:S62–S67
Hanze J, Eul BG, Savai R, Krick S, Goyal P, Grimminger F, Seeger W, Rose F (2003) RNA interference for HIF-1alpha inhibits its downstream signalling and affects cellular proliferation. Biochem Biophys Res Commun 312:571–577
Lall H, Coughlan K, Sumbayev VV (2008) HIF-1α protein is an essential factor for protection of myeloid cells against LPS-induced depletion of ATP and apoptosis that supports Toll-like receptor 4-mediated production of IL-6. Mol Immunol 45:3045–3049
Hart TW (1987) Some observation concerning the S-nitroso and S-phenylsulfonyl derivatives of L-cysteine and glutathione. Tetrahedron Lett 26:2013–2016
Sumbayev VV (2008) LPS-induced Toll-like receptor 4 signalling triggers cross-talk of apoptosis signal-regulating kinase 1 (ASK1) and HIF-1alpha protein. FEBS Lett 582:319–326
Sumbayev VV (2008) PI3 kinase and direct S-nitrosation are involved in down-regulation of apoptosis signal-regulating kinase 1 during LPS-induced Toll-like receptor 4 signalling. Immunol Lett 115:126–130
Sumbayev VV (2001) Activities of apoptotic signal 1-regulating protein kinase and poly-(ADP-ribose) polymerase and internucleosomal DNA fragmentation in rat liver during oxidative stress induced by cobalt chloride. Bull Exp Biol Med 131:119–120
Shatrov VA, Sumbayev VV, Zhou J, Brune B (2003) Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1alpha (HIF-1alpha) accumulation via redox-dependent mechanisms. Blood 101:4847–4849
Sumbayev VV, Sandau KB, Brune B (2002) Mesangial cells but not hepatocytes are protected against NO/O2 − cogeneration: mechanistic considerations. Eur J Pharmacol 444:1–11
Borutaite V, Brown G (2003) Nitric oxide induces apoptosis via hydrogen peroxide, but necrosis via energy and thiol depletion. Free Radical Biol Med 35:1457–1468
Acknowledgements
We thank Professor Hidenori Ichijo (University of Tokyo, Japan) and Dr. Tim Paget (Medway School of Pharmacy, University of Kent, UK) for the gift of reagents. This work was supported by the start-up grant provided by the Medway School of Pharmacy, University of Kent (United Kingdom).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jawahir, M., Nicholas, S.A., Coughlan, K. et al. Apoptosis signal-regulating kinase 1 (ASK1) and HIF-1α protein are essential factors for nitric oxide-dependent accumulation of p53 in THP-1 human myeloid macrophages. Apoptosis 13, 1410–1416 (2008). https://doi.org/10.1007/s10495-008-0267-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0267-9