Abstract
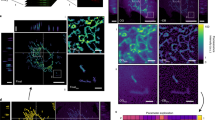
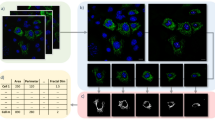
We have developed a versatile and rapid method for the quantitative estimation of cell death kinetics, following direct single-shot activation of the mitochondrial death pathway by a cell permeable BH3 activator peptide (D-R8BH3BID). This approach employs timelapse epifluorescent imaging of live cells and a machine- vision based feature extraction algorithm, to measure unidirectional stochastic transitions associated with mitochondrial inner membrane potential depolarization and/or permeability transition, at single cell resolution. This data is transformed to enable construction of a right step-wise survival function using the product limit estimator, and estimation of a median latency parameter (λ), defined for the entire imaged cell population. Estimates of λ computed for cells exhibiting two-colour fluorescence can be compared statistically using the Mantel-Hansel test. This general method has been applied to measure the kinetics and temporal ordering of BH3 domain induced mitochondrial depolarization and inner membrane permeabilization in cancer cells, and demonstrates the robustness of this technique in resolving temporally distinct intracellular events within individual cells.



Similar content being viewed by others
References
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2:156–162. doi:10.1038/35004029
Fennell DA, Pallaska A, Corbo M, Cotter FE (2005) Stochastic modelling of apoptosis kinetics. Apoptosis 10:447–452. doi:10.1007/s10495-005-0818-2
Mootha VK et al (2001) A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J 20:661–671. doi:10.1093/emboj/20.4.661
Nakagawa T et al (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658. doi:10.1038/nature03317
Schinzel AC et al (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010. doi:10.1073/pnas.0505294102
Baines CP et al (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662. doi:10.1038/nature03434
Wei MC et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730. doi:10.1126/science.1059108
Letai A et al (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183–192. doi:10.1016/S1535-6108(02)00127-7
Kuwana T et al (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525–535. doi:10.1016/j.molcel.2005.02.003
Petronilli V et al (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734
Minamikawa T, Williams DA, Bowser DN, Nagley P (1999) Mitochondrial permeability transition and swelling can occur reversibly without inducing cell death in intact human cells. Exp Cell Res 246:26–37. doi:10.1006/excr.1998.4290
Munoz-Pinedo C et al (2006) Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proc Natl Acad Sci USA 103:11573–11578. doi:10.1073/pnas.0603007103
Bernardi P (1992) Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem 267:8834–8839
Schonfeld P, Bohnensack R (1997) Fatty acid-promoted mitochondrial permeability transition by membrane depolarization and binding to the ADP/ATP carrier. FEBS Lett 420:167–170. doi:10.1016/S0014-5793(97)01511-1
Acknowledgements
The authors would like to thank Professor Peter Hamilton for helpful discussions; Arben Pallaska, Emma Hopkins for technical support. Dr Pat Harriott and Brett Greer for synthesis of BH3 peptides. D.A.F is a recipient of a Cancer Research UK Clinician Scientist Fellowship. A.C is supported by a Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chacko, A.D., Crawford, N.T., Johnston, P.G. et al. Machine vision based stochastic analysis of cancer cell mitochondrial dysfunction induced by a BH3 domain. Apoptosis 13, 1386–1393 (2008). https://doi.org/10.1007/s10495-008-0262-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0262-1