Abstract
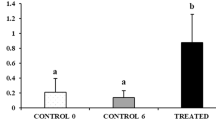
Using the simple cystic spermatogenesis in the shark testis as a model, we previously reported the relative resistance of immature spermatogonia (stem cell and early-stage spermatogonia) to apoptosis in the normal testis and after spermatoxicant exposure in vivo. Apoptosis was monitored by fluorescence image analysis of living cysts, using the validated acridine orange (AO) vital staining technique. Findings show that FBS simultaneously stimulates both apoptosis and [3H]thymidine incorporation in immature spermatogonial clones in a concentration-dependent manner in vitro. Furthermore, androgen inhibits apoptosis and increases cyst viability, more so with 10% FBS than with 1% FBS. All the effects were as a function of spermatogenic activity status but were distinct in early-stage spermatogonial cysts isolated from testes awakening from the previous winter spermatogenic arrest period. Results are discussed in the context of the alternating germ–Sertoli cell population kinetics of early-stage spermatogonial cysts in Squalus acanthias’s protracted testicular cycle.







Similar content being viewed by others
References
Griswold MD (1995) Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 52:211–216
Loir M, Sourdaine P, Mendis-Handagama SMLC, Jégou B (1995) Cell–cell interactions in the testis of teleosts and elasmobranchs. Microsc Res Tech 32:533–552
Steinberger E, Steinberger A (1975) Spermatogenic function of the testis. In: Hamilton DW, Greep RO (eds) Endocrinology vol V male reproductive system. American Physiological Society, Washington
Kerr JB (1995) Macro, micro, and molecular research on spermatogenesis: the quest to understand its control. Microsc Res Tech 32:364–384
Parvinen M (1982) Regulation of the seminiferous epithelium. Endocrine Rev 3:404–417
Kerr JB (1988) A light microscopic and morphometric analysis of the Sertoli cell during the spermatogenic cycle of the rat. Anat Embryol (Berl) 177:341–348
De Franca LR, Ghosh S, Ye S, Russell LD (1993) Surface and surface-to-volume relationships of the Sertoli cell during the cycle of the seminiferous epithelium in the rat. Biol Reprod 49:1215–1228
Sharpe RM (1994) Regulation of spermatogenesis. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven Press, New York
Timmons PM, Rigby PWJ, Poirer F (2002) The murine seminiferous epithelial cycle is pre-figured in the Sertoli cells of the embryonic testis. Development 129:635–647
Yoshida S, Sukeno M, Nakagawa T et al (2006) The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133:1495–1505
De Rooij DG, Janssen JM (1987) Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster: I. Undifferentiated spermatogonia. Anat Rec 217:124–130
Schlatt S, Zhengwei Y, Meehan T, De Kretser DM, Loveland KL (1999) Application of morphometric techniques to postnatal rat testes in organ culture: insights into testis growth. Cell Tiss Res 298:335–343
Boulogne B, Olaso R, Levacher C, Durand P, Habert R (1999) Apoptosis and mitosis in gonocytes of the rat testis during foetal and neonatal development. Int J Androl 22:356–365
McClusky LM (2005) Stage and season effects on cell cycle and apoptotic activities of germ cells and Sertoli cells during spermatogenesis in the spiny dogfish (Squalus acanthias). Reproduction 129:89–102
McClusky LM (2006) Stage-dependency of apoptosis and the blood-testis barrier in the dogfish shark (Squalus acanthias): cadmium-induced changes as assessed by vital fluorescence techniques. Cell Tiss Res 325: 541–553
McClusky LM (2008) Cadmium accumulation and binding characteristics in intact Sertoli/germ cell units, and associated effects on stage-specific functions in vitro: insights from a shark testis model. J Appl Toxicol 28: 112–121
Pudney J, Callard GV (1984) Identification of Leydig-like cells in the interstitium of the shark testis (Squalus acanthias). Anat Rec 209:311–330
DuBois W, Callard GV (1991) Culture of intact Sertoli/germ cells units and isolated Sertoli cells from Squalus testis: I. Evidence of stage-related functions in vitro. J Exp Zool 258:359–372
DuBois W, Callard GV (1993) Culture of intact Sertoli/germ cell units and isolated Sertoli cells from Squalus testis. II. Stimulatory effects of insulin and IGF-I on DNA synthesis in premeiotic stages. J Exp Zool 267:233–244
Cuevas ME, Callard GV (1992) In vitro secretion by staged spermatocysts (Sertoli/germ cell units) of dogfish (Squalus acanthias) testis. Gen Comp Endocrinol 88:151–165
Piferrer F, Callard GV (1995) Inhibition of deoxyribonucleic acid synthesis during premeiotic stages of spermatogenesis by a factor from testis-associated lymphomyeloid tissue in the dogfish shark (Squalus acanthias). Biol Reprod 53:390–398
Roosen-Runge EC (1977) The process of spermatogenesis in animals. Cambridge University Press, London
Abrams JM, White K, Fessler I (1993) Programmed cell death during Drosophila embryogenesis. Development 117:29–43
Durrieu F, Belloc F, Lacoste L et al (1998) Caspase activation is an early event in anthracycline-induced apoptosis and allows detection of apoptotic cells before they are ingested by phagocytes. Exp Cell Res 240:165–175
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Schwartz JR, Roy SK (1998) In vitro culture of hamster ovarian primary interstitial cells: effects of serum. Biol Reprod 59:1187–1194
Byrne AT, Southgate J, Brison DR, Leese HJ (1999) Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. Reproduction 117:97–105
Cui X, Jeong Y, Lee H, Cheon S, Kim N (2004) Fetal bovine serum influences apoptosis and apoptosis-related gene expression in porcine parthenotes developing in vitro. Reproduction 127:125–130
Bertolero F, Kaighn ME, Camalier RF, Saffiotti U (1986) Effects of serum and serum-derived factors on growth and differentiation of mouse keratinocytes. In vitro Cell Dev Biol 22:423–428
Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E (2006) Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol Prog 22:1294–1300
Kodiara K, Imada M, Goto M et al (2006) Purification and identification of a BMP-like factor from bovine serum. Biochem Biophys Res Comm 345:1224–1231
Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S (2003) Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci 116:3363–3372
Yu CL, Tsai MH (2001) Fetal fetuin selectively induces apoptosis in cancer cell lines and shows anti-cancer activity in tumor animal models. Cancer Lett 166:173–184
Inoue CN, Nagano I, Ichinohasama R, Asatao N, Kondo Y, Iinuma K (2001) Bimodal effects of platelet-derived growth factor on rat mesangial proliferation and death, and the role lysophosphatidic acid in cell survival. Clin Sci 101:11–19
Kuno G, Hink WF, Briggs JD (1971) Growth-promoting serum proteins for Aedes eagypti cells cultured in vitro. J Insect Physiol 17:1865–1873
Pinyopummintr T, Bavister BD (1994) Development of bovine embryos in a cell-free culture medium: effects of type of serum, timing of its inclusion and heat-inactivation. Theriogenology 41:1241–1249
Wesselborg S, Janssen O, Kabelitz D (1993) Induction of activation-driven death (apoptosis) in activated, but not resting peripheral blood T cells. J Immunol 150:4338–4345
Radvanyi LG, Shi Y, Mills GB, Miller RG (1996) Cell cycle progression out of G1 sensitizes primary-cultured nontransformed T cells to TCR-mediated apoptosis. Cell Immunol 170:260–273
Ruben LN, Johnson RO, Bergin A, Clothier RH (2000) Apoptosis and the cell cycle in Xenopus: PMA and MPMA exposure of splenocytes. Apoptosis 5:225–233
King KL, Cidlowski JA (1998) Cell cycle regulation and apoptosis. Ann Rev Physiol 60:601–617
Durrieu F, Samejima K, Fortune JM, Kandels-Lewis S, Osheroff N, Earnshaw WC (2000) DNA topoisomerase IIα interacts with nuclease and is involved in chromatin condensation during apoptotic execution. Curr Biol 10:923–926
Kuranaga E, Miura M (2007) Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol 17:135–144
Charvet CJ, Striedter GF (2008) Spatiotemporal clustering of cell death in the avian forebrain proliferative zone. Int J Dev Biol. doi:10.1387/ijdb.072455cc
Callard GV, Jorgensen JC, Redding JM (1995) Biochemical analysis of programmed cell death during premeiotic stages of spermatogenesis in vivo and in vitro. Dev Gen 16:140–147
Cuevas ME, Miller W, Callard GV (1992) Sulfoconjugation of steroids and the vascular pathway of communication in dogfish testis. J Exp Zool 264:119–129
Callard GV, Pudney JA, Mak P, Canick J (1985) Stage-dependent changes in steroidogenic enzymes and estrogen receptors during spermatogenesis in the testis of the dogfish Squalus acanthias. Endocrinology 117:1328–1335
Cuevas ME, Callard GV (1992) Androgen and progesterone receptors in shark (Squalus) testis: characteristics and stage-related distribution. Endocrinology 130:2173–2182
Sourdaine P, Garnier DH, Jégou B (1990) The adult dogfish (Scyliorhinus canicula L.) testis: a model to study stage-dependent changes in steroid levels during spermatogenesis. J Endocrinol 127:451–460
Henriksen K, Hakorvita H, Parvinen M (1995) Testosterone inhibits and induces apoptosis in rat seminiferous tubules in a stage-specific manner: in situ quantification in squash preparations after administration of ethane dimethane sulfonate. Endocrinology 136:3285–3291
Bruckheimer EM, Kypriano N (2001) Dihydrotestosterone enhances transforming growth factor-β-induced apoptosis in hormone-sensitive prostate cancer cells. Endocrinology 142:2419–2426
Walker WH, Cheng J (2005) FSH and testosterone signalling in Sertoli cells. Reproduction 130:15-28
Amsterdam A, Dantes A, Selvaraj N, Aharoni D (1997) Apoptosis in steroidogenic cells: structure–function analysis. Steroids 62:207–210
Loir M (1994) In vitro approach to the control of spermatogonia proliferation in the trout. Mol Cell Endocrinol 102:141–150
Loir M (1999) Spermatogonia of rainbow trout: II. In vitro study of the influence of pituitary hormones, growth factors and steroids on mitotic activity. Mol Reprod Dev 53:434–442
Loir M (1999) Spermatogonia of rainbow trout: III. In vitro study of the proliferative response to extracellular ATP and adenosine. Mol Reprod Dev 53:443–450
Dobson S, Dodd JM (1977) Endocrine control of the testis in the dogfish Scyliorhinus canicula L. II. Histological and ultrastructural changes in the testis after partial hypophysectomy (ventral lobectomy). Gen Comp Endocrinol 32:53–71
Dobson S, Dodd JM (1977) The roles of temperature and photoperiod in the response of the testis of the dogfish, Scyliorhinus canicula L. to partial hypophysectomy (ventral lobectomy). Gen Comp Endocrinol 32:114–115
Huckins C (1978) The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Rec 190:905–926
Russell LD, Clermont Y (1977) Degeneration of germ cells in normal, hypophysectomised and hormone treated hypophysectomised rats. Anat Rec 187:347–366
Acknowledgments
This research was carried out in part at the Mount Desert Island Biological Laboratory, Salisbury Cove, ME, USA and Boston University, Boston, MA, USA, and supported in part by a fellowship from the National Research Foundation of South Africa. Technical assistance provided by Andy Sexton (Marine Biological Laboratory, Woods Hole, MA, USA); Janet Fields and Vic Nordahl (National Marine Fisheries Service, Woods Hole, MA, USA); and Arnold Howe (Massachusetts Division of Marine Fisheries, Cape Cod Bay, MA, USA) in the collection of shark specimens is greatly appreciated. The author also wishes to thank Dr. David Miller (NIEHS) for his advice on fluorescence microscopy and Dr. Gloria Callard (Boston University) for helpful discussions during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClusky, L.M. Fetal bovine serum simultaneously stimulates apoptosis and DNA synthesis in premeiotic stages of spermatogenesis in spiny dogfish (Squalus acanthias) in vitro: modulation by androgen and spermatogenic activity status. Apoptosis 13, 649–658 (2008). https://doi.org/10.1007/s10495-008-0205-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0205-x