Abstract
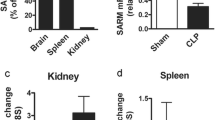
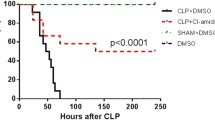
Suppressor of Cytokine Signaling (SOCS) proteins are recently identified inhibitors/regulators of cytokine/growth factor signaling pathways. We have previously shown that SOCS-3 is upregulated in mice after sepsis induced by cecal ligation and puncture; however, the contribution of SOCS-1 to septic morbidity and mortality is unclear. In the present study, we characterized SOCS-1 expression in different tissues and delineated putative mechanisms effecting SOCS-1 expression in thymus from septic mice. We observed no difference in SOCS-1 expression in blood, peritoneal leukocytes, lung, and spleen taken from sham or septic animals at 24 h after surgery. In contrast, SOCS-1 expression in thymus declined significantly after sepsis and this down-regulation of SOCS-1 was associated with increased thymocyte apoptosis as well as augmented Bax recruitment to the mitochondria. Administration of RU-38486, a steroid receptor antagonist, reversed the above effects in the septic thymus. Furthermore, SOCS-1+/− mice showed a significant higher mortality when compared to SOCS-1+/+ mice after sepsis. Together, these results show that sepsis increases steroid-induced thymic lymphoid cell apoptosis, which is associated with reduced SOCS-1 expression and increased Bax translocation to mitochondria. Survival data suggests that SOCS-1 protein may play an important role in sepsis.







Similar content being viewed by others
References
Alexander WS, Hilton DJ (2004) The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Ann Rev Immunol 22:503–529
Roberts AW, Robb L, Rakar S et al (2001) Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci USA 98:9324–9329
Takahashi Y, Carpino N, Cross JC et al (2003) SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J 22:372–384
Starr R, Metcalf D, Elefanty AG et al (1998) Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA 95:14395–14399
Naka T, Matsumoto T, Narazaki M et al (1998) Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci USA 95:15577–15582
Alexander WS (2002) Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2:410–416
Tan JC, Rabkin R (2005) Suppressors of cytokine signaling in health and disease. Pediatr Nephrol 20:567–575
Kubo M, Hanada T, Yoshimura A (2003) Suppressors of cytokine signaling and immunity. Nature Immunol 4:1169–1176
Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ (2004) Re-examination of the Role of Suppressor of Cytokine Signaling 1 (SOCS1) in the Regulation of Toll-like Receptor Signaling. J Biol Chem 279:54702–54707
Baetz A, Frey M, Heeg K, Dalpke AH (2004) Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem 279:54708–54715
Kimura A, Naka T, Nagata S, Kawase I, Kishimoto T (2004) SOCS-1 suppresses TNF-a-induced apoptosis through the regulation of Jak activation. Int Immunol 16:991–999
Ogle CK, Kong F, Guo X et al (2000) The effect of burn injury on suppressors of cytokine signalling. Shock 14:392–399
Grutkoski PS, Chen Y, Chung CS, Cioffi WG, Ayala A (2004) Putative mechanism of hemorrhage-induced leukocyte hyporesponsiveness:induction of suppressor of cytokine signaling-3. J Trauma 56:742–748
Johnson TS, O’Leary M, Justice SK et al (2001) Differential expression of suppressors of cytokine signalling genes in response to nutrition and growth hormone in the septic rat. J Endocrinology 169:409–415
Hong-Brown LQ, Brown CR, Cooney RN, Frost RA, Lang CH (2003) Sepsis-induced muscle growth hormone resistance occurs independently of STAT5 phosphorylation. Am J Physiol Endocrinol Metab 285:E63–E72
Grutkoski PS, Chen Y, Chung CS, Ayala A (2003) Sepsis-induced SOCS-3 expression is immunologically restricted to phagocytes. J Leukoc Biol 74:916–922
Ayala A, Herdon CD, Lehman DL et al (1995) The induction of accelerated thymic programmed cell death during polymicrobial sepsis: Control by corticosteroids but not tumor necrosis factor. Shock 3:259–267
Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH (1992) Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophage to release inflammatory mediators (IL-1, IL-6 and TNF). Circ Shock 36:191–199
Ayala A, Perrin MM, Wagner MA, Chaudry IH (1990) Enhanced susceptibility to sepsis following simple hemorrhage: Depression of Fc and C3b receptor mediated phagocytosis. Arch Surg 125:70–75
Meldrum DR, Ayala A, Perrin MM, Ertel W, Chaudry IH (1991) Diltiazem restores IL-2, IL-3, IL-6 and IFN-gamma synthesis and decreases susceptibility to sepsis following hemorrhage. J Surg Res 51:158–164
Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH (1996) Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood 87:4261–4275
Chung CS, Song GY, Moldawer LL, Chaudry IH, Ayala A (2000) Neither Fas ligand nor endotoxin is responsible for inducible phagocyte apoptosis during sepsis/peritonitis. J Surg Res 91:147–153
Garvy BA, Telford WG, King LE, Fraker PJ (1993) Glucocorticoids and irradiation-induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology 79:270–277
Stocklin E, Wissler M, Gouilleux F, Groner B (1996) Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728
Bianchi M, Meng C, Ivashkiv LB (2000) Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci USA 97:9573–9578
Hyde SR, McCallum RE (1992) Lipopolysaccharide-tumor necrosis factor-glucocorticoid interactions during cecal ligation and puncture-induced sepsis in mature versus senescent mice. Infect Immun 60:976–982
Yoshino T, Kishi H, Nagata T et al (2001) Differential involvement of p38 MAP kinase pathway and Bax translocation in the mitochondria-mediated cell death in TCR- and dexamethasone-stimulated thymocytes. Eur J Immunol 31:2702–2708
Ashwell JD, Frank WM, Vacchio MS (2000) Glucocorticoids in T Cell Development and Function. Annu Rev Immunol 18:309–345
Greenhalgh CJ, Hilton DJ (2001) Negative regulation of cytokine signaling. J Leukoc Biol 70:348–356
Hotchkiss RS, Tinsley KW, Swanson PE et al (1999) Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA 96:14541–14546
Deitch EA (1998) Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11
Starr R, Willson TA, Viney EM et al (1997) A family of cytokine-inducible inhibitors of signaling. Nature 387:917–921
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2 -deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229–240
Brady HJ, Salomaons GS, Bobeldijk RC, Berns AJ (1996) T cells from baxalpha transgenic mice show accelerated apoptosis in response to stimuli but do not show restored DNA damage-induced cell death in the absence of p53 gene product in. EMBO J 15:1221–1230
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Goping IS, Gross A, Navoie JN et al (1998) Regulated targeting of BAX to mitochondria. J Cell Biol 143:207–215
Hoijman E, Viegas LR, Sarmiento MIK, Rosenstein RE, Pecci A (2004) Involvement of Bax protein in the prevention of glucocorticoid-induced thymocytes apoptosis by melatonin. Endocrinology 145:418–425
Morita Y, Naka T, Kawazoe Y et al (2000) Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor a-induced cell death in fibroblasts. PNAS 97:5405–5410
Fukada T, Hibi M, Yamanaka Y et al (1996) Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5:449–460
Quelle FW, Wang J, Feng J et al (1998) Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev 12:1099–1107
Ilangumaran S, Rottapel R (2003) Regulation of cytokine receptor signaling by SOCS1. Immunological Reviews 192:196–211
Hansen JA, Lindberg K, Hilton DJ, Nielson JH, Billestrup N (1999) Mechanism of inhibition of growth hormone receptor signaling by suppressor of cytokine signaling proteins. Mol Endrocrinology 13:1832–1843
Zhang Y-H, Takahashi K, Jiang G-Z et al (1993) In vivo induction of apoptosis (programmed cell death) in mouse thymus by administration of lipopolysaccharide. Infect Immun 61:5044–5048
Kato Y, Morikawa A, Sugiyama T et al (1997) Augmentation of lipopolysaccharide-induced thymocyte apoptosis by interferon-gamma. Cell Immunol 177:103–108
Nakagawa R, Naka T, Tsutsui H et al (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687
Kinjyo I, Hanada T, Inagaki-Ohara K et al (2002) SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591
Lindeman GJ, Wittlin S, Lada H et al (2001) SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev 15:1631–1636
Fujimoto M, Tsutsui H, Yumikura-Futatsugi S et al (2002) A regulatory role for suppressor of cytokine signaling-1 in Th polarization in vivo. Int Immunol 14:1343–1350
Hotchkiss RS, Swanson PE, Knudson CM et al (1999) Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 162:4148–4156
Acknowledgments
This work was supported by NIH R01-GM46354, NIH R01-GM53209 (A.A.) and the Lifespan Developmental Grants (C.C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, CS., Chen, Y., Grutkoski, P.S. et al. SOCS-1 is a central mediator of steroid-increased thymocyte apoptosis and decreased survival following sepsis. Apoptosis 12, 1143–1153 (2007). https://doi.org/10.1007/s10495-007-0059-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0059-7