Abstract
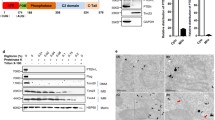
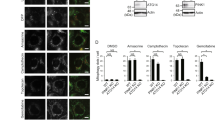
Apoptosis is a genetically determined cell suicide program. Mitochondria play a central role in this process and various molecules have been shown to regulate apoptosis in this organelle. In the present study, we firstly identified that protein tyrosine phosphatase interacting protein 51 (PTPIP51) is a novel mitochondrial protein, which may induce apoptosis in HEK293T and HeLa cell lines. PTPIP51 transfection resulted in the externalization of phosphatidylserine (PS), activation of caspase-3, cleavage of PARP, and condensation of nuclear DNA. Further investigation revealed that PTPIP51 over-expression caused a decrease in mitochondrial membrane potential and release of cytochrome c, suggesting that it may be involved in a mitochondria/cytochrome c mediated apoptosis pathway. We also found that a putative TM domain near the N terminus of PTPIP51 is required for its targeting to mitochondria, as evidenced by the finding that deletion of the PTPIP51 TM domain prevented the protein's mitochondiral localization. Furthermore, this deletion significantly influenced the ability of PTPIP51 to induce apoptosis. Taken together, the results of the present study suggest that PTPIP51 is a mitochondrial protein with apoptosis-inducing function and that the N-terminal TM domain is required for both the correct targeting of the protein to mitochondria and its apoptotic functions.
Similar content being viewed by others
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8:115–128
Thornberry NA, Lazebnik Y (1998) Caspases: Enemies within. Science 281:1312–1316
Ashkenazi A (2002) Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2:420–430
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2-interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Gross A, Yin XM, Wang K et al (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem 274:1156–1163
Li P, Nijhawan D Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Zou H, Li Y, Liu X, Wang X (1999) An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377
Paquet C, Schmitt E, Beauchemin M, Bertrand R (2004) Activation of multidomain and BH3-only pro-apoptotic Bcl-2 family members in p53-defective cells. Apoptosis 9:815–831
Park BS, Song YS, Yee SB et al (2005) Phospho-ser 15-p53 translocates into mitochondria and interacts with Bcl-2 and Bcl-xL in eugenol-induced apoptosis. Apoptosis 10:193–200
Marchenko ND, Zaika A, Moll UM (2000) Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 275:16202–16212
Perfettini JL, Roumier T, Kroemer G (2005) Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol 15:179–183
Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15:5001–5011
Stenzinger A, Kajosch T, Tag C et al (2005) The novel protein PTPIP51 exhibits tissue- and cell-specific expression. Histochem Cell Biol 123:19–28
Wang L, Gao X, Gao P et al (Jan 4, 2006) Cell-based screening and validation of human novel genes associated with cell viability. J Biomol Screen. Accepted for publication
Lv B, Shi T, Wang X et al (2006) Overexpression of the novel human gene, nuclear apoptosis-inducing factor 1, induces apoptosis. Int J Biochem Cell Bio 38:671–683
Wang Y, Li X, Wang L et al (2004) An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci 117:1525–1532
Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM (2003) The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem 278:11633–11641
Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC (1993) Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem 268:25265–25268
James DI, Parone PA, Mattenberger Y, Martinou JC (2003) hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278:36373–36379
Nicholls DG (2002) Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol 34:1372–1381
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A 91:10771–10778
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, B.F., Yu, C.F., Chen, Y.Y. et al. Protein tyrosine phosphatase interacting protein 51 (PTPIP51) is a novel mitochondria protein with an N-terminal mitochondrial targeting sequence and induces apoptosis. Apoptosis 11, 1489–1501 (2006). https://doi.org/10.1007/s10495-006-8882-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-8882-9