Abstract
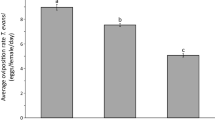
Several species of tetranychid mites including Tetranychus urticae Koch (Acari: Tetranychidae) construct complicated three-dimensional webs on plant leaves. These webs provide protection against biotic and abiotic stress. As producing web is likely to entail a cost, mites that arrive on a leaf with web are expected to refrain from producing it, because they will gain the benefit of protection from the existing web. Mites that produce less web may then allocate resources that are not spent on web construction to other fitness-enhancing activities, such as laying eggs. To test this, the oviposition rate of T. urticae adult females was examined on leaves with web. As a control, we used leaves where the web had been removed, hence both types of leaves had been exposed to conspecifics previously and were thus damaged. On leaves with web, the oviposition rate of T. urticae females was higher than on leaves where the web had been removed. Therefore, the presence of web constructed by conspecifics enhanced the oviposition rate of T. urticae females. This provides indirect evidence that mites use the web constructed by conspecifics and thereby save resources that can be allocated to other traits that enhance reproductive success.


Similar content being viewed by others
References
Allee W, Emerson O, Park T, Schmidt K (1949) Principles of animal ecology. W.B. Saunders, Philadelphia
Davis DW (1952) Influence of population density on Tetranychus multisetis. J Econ Entomol 45:652–654
Denno RF, Benrey B (1997) Aggregation facilitates larval growth in the neotropical nymphalid butterfly Chlosyne janais. Ecol Entomol 22:133–141. doi:10.1046/j.1365-2311.1997.t01-1-00063.x
Despland E, Le Huu A (2007) Pros and cons of group living in the forest tent caterpillar: separating the roles of silk and of grouping. Entomol Exp Appl 122:181–189. doi:10.1111/j.1570-7458.2006.00512.x
Furuichi H, Oku K, Yano S, Takafuji A, Osakabe M (2005) Why does the predatory mite Neoseiulus womersleyi Schicha (Acari: Phytoseiidae) prefer spider mite eggs to adults? Appl Entomol Zool (Jpn) 40:675–678. doi:10.1303/aez.2005.675
Gerson U (1985) Webbing. In: Sabelis MW, Helle W (eds) Spider mites. Their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 223–232
Gols R, Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol 29:2651–2666. doi:10.1023/B:JOEC.0000008010.40606.b0
Gotoh T (1997) Annual life cycles of populations of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) in four Japanese pear orchards. Appl Entomol Zool (Jpn) 32:207–216
Gotoh T, Kaibara S, Tamura I (2007) Species composition and seasonal changes of spider mite density on a leguminous plant Pueraria lobata. Appl Entomol Zool (Jpn) 42:685–692. doi:10.1303/aez.2007.685
Hazan A, Gertler A, Tahori AS, Gerson U (1975) Spider mite webbing III. Solubilization and amino acid composition of the silk protein. Comp Biochem Physiol 51B:457–462
Maeda T, Takabayashi J (2001) Production of herbivore-induced plant volatiles and their attractiveness to Phytoseius persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (Acari: Tetranychidae) density on a plant. Appl Entomol Zool (Jpn) 36:47–52. doi:10.1303/aez.2001.47
Magalhães S, Janssen A, Montserrat M, Sabelis MW (2005) Host-plant species modifies the diet of an omnivore feeding on three trophic levels. Oikos 111:47–56. doi:10.1111/j.0030-1299.2005.13897.x
Magalhães S, van Rijn PCJ, Montserrat M, Pallini A, Sabelis MW (2007) Population dynamics of thrips prey and their mite predators in a refuge. Oecologia 150:557–568. doi:10.1007/s00442-006-0548-3
McMurtry JA, Huffaker CB, van de Vrie M (1970) Ecology of tetranychid mites and their natural enemies: a review I. Tteranychid enemies: their biological characters and the impact of spray practices. Hilgardia 40:331–390
Mori K, Saito Y, Sakagami T (1999) Effects of the nest web and female attendance on survival of young in the subsocial spider mite Schizotetranychus longus (Acari: Tetranychidae). Exp Appl Acarol 23:411–418. doi:10.1023/A:1006165606428
Oku K (2008) Role of excreta in predator avoidance by the Kanzawa spider mite, Tetranychus kanzawai (Acari: Tetranychidae). Eur J Entomol 105:619–623
Oku K, Yano S, Takafuji A (2002) Different maternal effects of on offspring performance in tetranychid mites, Tetranychus kanzawai and T. urticae (Acari: Tetranychidae). Appl Entomol Zool (Jpn) 37:425–429. doi:10.1303/aez.2002.425
Oku K, Yano S, Takafuji A (2003) Spider mite’s use of a refuge during the quiescent stage in the presence of a predator. Entomol Exp Appl 108:71–74. doi:10.1046/j.1570-7458.2003.00069.x
Oku K, Yano S, Takafuji A (2004) Nonlethal indirect effects of a native predatory mite, Amblyseius womersleyi Schicha (Acari: Phytoseiidae), on the phytophagous mite Tetranychus kanzawai Kishida (Acari: Tetranychidae). J Ethol 22:109–112. doi:10.1007/s10164-003-0102-2
Roda A, Nyrop J, Dicke M, English-Loeb G (2000) Trichomes and spider-mite webbing protect predatory mite eggs from intraguild predation. Oecologia 125:428–435. doi:10.1007/s004420000462
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The Acari: reproduction. Development and life-history strategies. Chapman & Hall, London, pp 23–50
Saito Y (1979) Study on spinning behaviour of spider mites III. Response of mites to webbing residues and their preferences for particular physical conditions of leaf surfaces (Acarina: Tetranychidae). Jap J Appl Entomol Zool 23:82–91 in Japanese with an English summary
Saito Y (1983) The concept of “life types” in Tetranychinae. An attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–391
Takafuji A, Kamibayashi M (1984) Life cycle of a non-diapausing population of the two-spotted spider mite, Tetranychus urticae Koch in a pear orchard. Res Popul Ecol (Kyoto) 26:113–123. doi:10.1007/BF02515511
Trichilo PJ, Leigh TF (1986) Predation on spider mite eggs by the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), an opportunist in a cotton agroecosystem. Environ Entomol 15:821–825
Viscido SV, Wethey DS (2002) Quantitative analysis of fiddler crab flock movement: evidence for ‘selfish herd’ behaviour. Anim Behav 63:735–741. doi:10.1006/anbe.2001.1935
Watt PJ, Nottingham SF, Young S (1997) Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Anim Behav 54:865–872. doi:10.1006/anbe.1996.0512
Wertheim B, van Balen EJA, Dicke M, Vet LEM (2005) Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu Rev Entomol 50:321–346. doi:10.1146/annurev.ento.49.061802.123329
Yano S, Wakabayashi M, Takabayashi J, Takafuji A (1998) Factors determining the host plant range of the phytophagous mite, Tetranychus urticae (Acari: Tetranychidae): a method for quantifying host plant acceptance. Exp Appl Acarol 22:595–601. doi:10.1023/A:1006138527904
Acknowledgments
We are grateful to R. Gols and two anonymous reviewers for valuable suggestions. We also thank L. Koopman, F. van Aggelen and A. Gidding for rearing mites and plants. This study was partly supported by a subsidy from the Japan Society for the Promotion of Science for Young Scientists (no. 4537) to K.O.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oku, K., Magalhães, S. & Dicke, M. The presence of webbing affects the oviposition rate of two-spotted spider mites, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 49, 167–172 (2009). https://doi.org/10.1007/s10493-009-9252-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-009-9252-4