Abstract
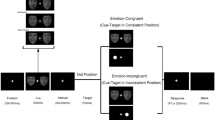
There appears to be a significant disconnect between symptomatic and functional recovery in bipolar disorder (BD). Some evidence points to interepisode cognitive dysfunction. We tested the hypothesis that some of this dysfunction was related to emotional reactivity in euthymic bipolar subjects may effect cognitive processing. A modification of emotional gender categorization oddball task was used. The target was gender (probability 25%) of faces with negative, positive, and neutral emotional expression. The experiment had 720 trials (3 blocks × 240 trials each). Each stimulus was presented for 150 ms, and the EEG/ERP responses were recorded for 1,000 ms. The inter-trial interval was varied in 1,100–1,500 ms range to avoid expectancy effects. Task took about 35 min to complete. There were 9 BD and 9 control subjects matched for age and gender. Reaction time (RT) was globally slower in BD subjects. The centro-parietal amplitudes at N170 and N200, and P200 and P300 were generally smaller in the BD group compared to controls. Latency was shorter to neutral and negative targets in BD. Frontal P200 amplitude was higher to emotional negative facial non-targets in BD subjects. The frontal N200 in response to positive facial emotion was less negative in BD subjects. The frontal P300 of BD subjects was lower to emotionally neutral targets. ERP responses to facial emotion in BD subjects varied significantly from normal controls. These variations are consistent with the common depressive symptomology seen in long term studies of bipolar subjects.



Similar content being viewed by others
References
Addington, J., & Addington, D. (1997). Attentional vulnerability indicators in schizophrenia and bipolar disorder. Schizophrenia Research, 23, 197–204.
Adolphs, R. (1996). Cortical systems for the recognition of emotion in facial expressions. Journal of Neuroscience, 16, 328–333.
Adolphs, R. (2002). Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews, 1(1), 21–62.
Ahern, G. L., Schomer, D. L., Kleefield, J., Blume, H., Cosgrove, G. R., Weintraub, S., et al. (1991). Right hemisphere advantage for evaluating emotional facial expressions. Cortex, 27, 193–202.
Amado-Boccara, I., Gougoulis, N., Poirier-Littre, M. F., Galinowski, A., & Loo, H. (1995). Effects of antidepressants on cognitive functions: A review. Neuroscience and Biobehavioral Reviews, 19, 479–493.
An, S. K., Lee, S. J., Lee, S. H., Cho, H. S., Lee, P. G., Lee, C., et al. (2003). Reduced P3 amplitudes by negative facial emotional photographs in schizophrenia. Schizophrenia Research, 64, 125–135.
Atre-Vaidya, N., Taylor, M., Seidenberg, M., Reed, R., Perrine, A., & Glick-Oberwise, F. (1998). Cognitive deficits, psychopathology and psychosocial functioning in bipolar mood disorder. Neuropsychiatry, Neuropsychology and Behavioral. Neurology, 11, 120–126.
Austin, M., Mitchell, P., & Goodwin, G. M. (2001). Cognitive deficits in depression: Possible implications for functional neuropathology. British Journal of Psychiatry, 178, 200–206.
Balconi, M., & Pozzoli, U. (2003). Face-selective processing and the effect of pleasant and unpleasant emotional expressions on ERP correlates. International Journal of Psychophysiology, 49, 67–74.
Bange, F., & Bathien, N. (1998). Visual cognitive dysfunction in depression: An event-related potential study. Electroencephalography and Clinical Neurophysiology, 108, 472–481.
Batty, M., & Taylor, M. J. (2003). Early processing of the six basic facial emotional expressions. Brain Research: Cognitive Brain Research, 17(3), 613–620.
Bearden, C. E., Hoffman, K. M., & Canon, T. D. (2001). The neuropsychology and neuroanatomy of bipolar affective disorder: A critical review. Bipolar Disorder, 3, 106–150.
Beats, B. C., Sahakian, B. J., & Levy, R. (1996). Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychology Medicine, 26, 591–603.
Bentin, S., Allison, T., Puce, A., Perez, E., & McCarthy, G. (1996). Electrophysiological studies of face perception in human. Journal of Cognitive Neuroscience, 8, 551–565.
Bigelow, L. B., & Berthot, B. D. (1989). The Psychiatric Symptom Assessment Scale (PSAS). Psychopharmacology Bulletin, 25, 168–173.
Blumberg, H. P., Stem, E., Martinez, D., Ricketts, S., de Asis, J., White, T., et al. (2000). Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry, 48, 1045–1052.
Blumberg, H. P., Stern, E., Martinez, D., Ricketts, S., de Asis, J., White, T., et al. (1999). Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. American Journal of Psychiatry, 156, 1986–1988.
Borkowska, A., & Rybakowski, J. K. (2001). Neuropsychological frontal lobe tests indicate that bipolar depressed patients are more impaired than unipolar. Bipolar Disorder, 3, 88–94.
Bozikas, V. P., Tonia, T., Fokas, K., Karavatos, A., & Kosmidis, M. H. (2006). Impaired emotion processing in remitted patients with bipolar disorder. Journal of Affective Disorders, 91, 53–56.
Bramon, E., Rabe-Hesketh, S., Sham, P., Murray, R. M., & Frangou, S. (2004). Meta-analysis of the P300 and P50 in schizophrenia. Schizophrenia Research, 70, 315–329.
Brazdil, M., Rektor, I., Daniel, P., Dufek, M., & Jurak, P. (2001). Intracerebral event-related potentials to subthreshold target stimuli. Clinical Neurophysiology, 112, 650–661.
Bremner, J. D., Vermetten, E., Vythilingam, M., Afzal, N., et al. (2004). Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry, 55, 612–620.
Bruder, G. E., Tenke, C. E., Towey, J. P., Leite, P., Fong, R., Stewart, J. E., et al. (1998). Brain ERPs of depressed patients to complex tones in an oddball task: Relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology, 35, 54–63.
Caligiuri, M. P., & Ellwanger, J. (2000). Motor and cognitive aspects of motor retardation in depression. Journal of Affective Disorders, 57, 83–93.
Campanella, S., Quinet, P., Bruyer, R., Crommelinck, M., & Guérit, J. M. (2002). Categorical perception of happiness and fear facial expressions: An ERP study. Journal of Cognitive Neuroscience, 14, 210–227.
Campanella, S., Rossignol, M., Mejias, S., Joassin, F., Maurage, P., Debatisse, D., et al. (2004). Human gender differences in an emotional visual oddball task: An event-related study. Neuroscience Letters, 367, 14–18.
Carretié, L., Mercado, F., Hinojosa, J., Martin-Loeches, M., & Sotillo, M. (2004). Valence-related vigilance biases in anxiety studied through event-related potentials. Journal of Affective Disorders, 78, 119–130.
Chengappa, K. N., Hennen, J., Baldessarini, R. J., Kupfer, D. J., Yatham, L. N., Gershon, S., et al. (2005). Recovery and functional outcomes following olanzapine treatment for bipolar I mania. Bipolar Disorder, 7, 68–76.
Clark, L., Iversen, S. D., & Goodwin, G. M. (2002). Sustained attention deficit in bipolar disorder. British Journal of Psychiatry, 180, 313–319.
Condray, R., Steinhauer, S. R., Cohen, J. D., van Kammen, D. P., & Kasparek, A. (1999). Modulation of language processing in schizophrenia: Effects of cortex and haloperidol on the event-related potential. Biological Psychiatry, 45, 1336–1355.
Conus, P., Cotton, S., Abdel-Baki, A., Lamvert, M., Berk, M., & McGorry, P. D. (2006). Symptomatic and functional outcome 12 months after a first episode of psychotic mania: Barriers to recovery in a catchment area sample. Bipolar Disorder, 8, 221–231.
Davidson, R. J., Lewis, D. A., Alloy, L. B., Amaral, D., Bush, G., Cohen, J., et al. (2002). Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry, 52, 478–502.
Deldin, P., Denevey, C. M., Kim, A. S., Casas, B., & Best, J. L. (2001a). A slow wave investigation of working memory biases in mood disorders. Journal of Abnormal Psychology, 110, 267–281.
Deldin, P. J., Keller, J., Gergen, J. A., & Miller, G. A. (2001b). Cognitive bias and emotion in neuropsychological models of depression. Cognition and Emotion, 15, 787–802.
Dietrich, D. E., Emrich, H. M., Waller, C., Wieringa, B. M., Johannes, S., & Munte, T. F. (2000). Emotion/cognition in word recognition memory of depressive patients: An event-related potential study. Psychiatry Research, 96, 15–29.
Donkers, F. C. L., & van Boxtel, G. J. M. (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition, 56, 165–176.
Eimer, M., & Holmes, A. (2007). Event-related brain potential correlates of emotional face processing. Neuropsychologia, 45, 15–31.
Elliott, R., Rubinsztein, J. S., Sahakian, B. J., & Dolan, R. J. (2002). The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry, 59, 597–604.
Ferrier, I. N., Stanton, B. R., Kelly, T. P., & Scott, J. (1999). Neuropsychological function in euthymic patients with bipolar disorder. British Journal of Psychiatry, 175, 246–251.
Ferrier, I. N., & Thompson, J. M. (2002). Cognitive impairment in bipolar affective disorder: Implications for the bipolar diathesis. British Journal of Psychiatry, 180, 293–295.
Ford, J. M., White, P. M., Csernansky, J. G., Faustman, W. O., Roth, W. T., & Pfefferbaum, A. (1994). ERPs in schizophrenia: Effects of antipsychotic medication. Biological Psychiatry, 36, 153–170.
Friedman, D., Simpson, G. V., & Hamberger, M. (1993). Age-related changes in scalp topography to novel and target stimuli. Psychophysiology, 30, 383–396.
Gangadhar, B. N., Ancy, J., Janakiramaiah, N., & Umapathy, C. (1993). P300 amplitude in non-bipolar, melancholic depression. Journal of Affective Disorders, 28, 57–60.
Gonul, A. S., Suer, C., Coburn, K., Ozesmi, C., Oguz, A., & Yilmaz, A. (2003). Effects of olanzapine on auditory P300 in schizophrenia. Progress in Neuro-Pharmacology Behavioral Psychiatry, 27, 173–177.
Goodwin, G. M., Cavanagh, J., Glabus, M., Kehoe, R., O’Carroll, R. E., & Ebmeier, K. P. (1997). Uptake of 99 mTc-exametazime shown by single photon emission computed tomography before and after lithium withdrawal in bipolar patients: Associations with mania. British Journal of Psychiatry, 170, 426–430.
Goodwin, F. K., & Ghaemi, S. N. (1988). Understanding manic–depressive illness. Archives of General Psychiatry, 55, 23–25.
Gorno-Tempini, M. L., Pradelli, S., Serafini, M., Pagnoni, G., Baraldi, P., Porro, C., et al. (2001). Explicit and incidental facial expression processing: An fMRI study. Neuroimage, 14, 465–473.
Goswami, U., Sharma, A., Khastigir, U., Ferrier, I. N., Young, A. H., Gallagher, P., et al. (2006). Neuropsychological dysfunction, soft neurological signs and social disability in euthymic patients with bipolar disorder. British Journal of Psychiatry, 188, 366–373.
Gur, R. G., Erwin, R. J., Gur, R. E., Zwil, A., Heimberg, C., & Kraemer, H. C. (1992). Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research, 42, 241–251.
Halit, H., de Haan, M., & Johnson, M. H. (2000). Modulation of event-related potentials by prototypical and atypical faces. NeuroReport, 11, 1871–1875.
Hansenne, M., Pitchot, W., Gonzalez-Moreno, A., Gonzalez-Torrecilas, J., Mirel, J., & Ansseau, M. (1994). Psychophysiological correlates of suicidal behavior in depression. A preliminary study. Neuropsychobiology, 30, 1–3.
Harmer, C. J., Grayson, L., & Goodwin, G. M. (2002). Enhanced recognition of disgust in bipolar illness. Psychiatry Research, 51, 298–304.
Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2000). The disturbed human neural system for face perception. Trends in Cognitive Sciences, 4(6), 223–233.
Holmes, A., Nielsen, M. K., & Green, S. (2008). Effects of anxiety on the processing of fearful and happy faces: An event-related potential study. Biological Psychology, 77(2), 159–173.
Jeon, Y.-W., & Polich, J. (2003). Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology, 40, 684–701.
Judd, L. L., Akiskal, H. S., Schettler, P. J., Coryell, W., Endicott, J., Maser, J. D., et al. (2003). A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Archives of General Psychiatry, 60, 261–269.
Judd, L. L., Akiskal, H. S., Schettler, P. J., Endicott, J., Maser, J., Solomon, D. A., et al. (2002). The long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of General Psychiatry, 59, 530–537.
Katayama, J., & Polich, J. (1998). Stimulus context determines P3a and P3b. Psychophysiology, 35, 23–33.
Kayser, J., Bruder, G. F., Tenke, C. E., Stewart, J. W., & Quitkin, P. M. (2002). Event-related potentials (ERPs) to hemifield presentation of emotional stimuli. International Journal of Psychophysiology, 36, 211–236.
Kesler-West, M. L., Andersen, A. H., Smith, C. D., Avison, M. J., Davis, C. E., Kryscio, R. J., et al. (2001). Neural substrates of facial emotion processing using fMRI. Brain Research: Cognitive Brain Research, 11, 213–226.
Krolak-Salmon, P., Fischer, C., Vighetto, A., & Mauguiere, F. (2001). Processing of facial emotional expression: Spatio-temporal data as assessed by scalp event-related potentials. European Journal of Neuroscience, 13, 987–994.
Laes, J. R., & Sponheim, S. R. (2006). Does cognition predict community function only in schizophrenia? A study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophrenia Research, 84, 121–131.
Lewis, S., & Garver, D. (1995). Treatment and diagnostic subtype in facial affect recognition in schizophrenia. Journal of Psychiatry Research, 29, 5–11.
Lyon, H. M., Startup, M., & Bentall, R. P. (1999). Social cognition and the manic defense: Attributions, selective attention, and self-schema in bipolar affective disorder. Journal of Abnormal Psychology, 108, 273–282.
MacQueen, G. M., Young, L. T., & Joffe, R. T. (2001). A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatrica Scandinavia, 103, 163–170.
Malhi, G. S., Lagopoulos, J., Sachdev, P. S., Ivanovski, B., & Shnier, R. (2005). An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disorder, 7(suppl 5), 58–69.
Martinez-Aran, A., Penades, R., Vieta, E., Colom, F., Reinares, M., Benabarre, A., et al. (2002). Executive function in patients with remitted bipolar disorder and schizophrenia and its relationship with functional outcome. Psychotherapy and Psychosomatics, 71, 39–46.
Martinez-Aran, A., Vieta, E., Reinares, M., Colom, F., Torrent, C., Sanchez-Moreno, J., et al. (2004). Cognitive function across manic or hypomanic, depressed and euthymic states in bipolar disorder. American Journal of Psychiatry, 161, 262–270.
Matsumoto, D., & Ekman, P. (2004). Japanese and Caucasian expressions of emotion (JACFEE) and neutral faces (JACNeuf). CA: Berkeley.
Mayberg, H. S. (1997). Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences, 9, 471–481.
Mayberg, H. S., Liotti, M., Brannan, S. K., McGinnis, S., Mahurin, R. K., Jarabek, P. A., et al. (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156, 675–682.
McCarthy, G., Puce, A., Belger, A., & Allison, T. (1999). Electrophysiological studies of human face perception. II. Response properties of face-specific potentials generated in occipito-temporal cortex. Cerebal Cortex, 9, 431–444.
McGrath, J., Scheldt, S., Welham, J., & Clair, A. (1997). Performance on tests sensitive to impaired executive ability in schizophrenia, mania and well controls: Acute and subacute phases. Schizophrenia Research, 26, 127–137.
Montgomery, S. A., & Asberg, M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–389.
Morris, J. S., Öhman, A., & Dolan, R. J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467–470.
Mueser, K. T., Penn, D. L., Blanchard, J. J., & Bellack, A. S. (1997). Affect recognition in schizophrenia: A synthesis of findings across three studies. Psychiatry, 60, 301–308.
Muir, W. J., St. Clair, D. M., & Blackwood, D. H. (1991). Long-latency auditory event related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychology Medicine, 21, 867–879.
Munte, T. F., Brack, M., Grootheer, O., Wieringa, B. M., Matzke, M., & Johannes, S. (1998). Brain potentials reveal the timing of face identity and expression judgments. Neuroscience Research, 30, 25–34.
Murphy, F. C., Sahakian, B. J., Rubinsztein, J., Michael, A., Rogers, R. D., Robbins, T. W., et al. (1999). Emotional bias and inhibitory control processes in mania and depression. Psychology Medicine, 29, 1307–1321.
Näätänen, R. A. (1990). The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive functioning. Behavioral Brain Science, 13, 201–287.
Näätänen, R. A., Gaillard, W. K., & Mäntysalo, S. (1978). Early selective attention effect on evoked potential reinterpreted. Acta Psychologica, 2, 313–329.
Nehra, R., Chakrabarti, S., Pradhan, B. K., & Khehra, N. (2006). Comparison of cognitive functions between first- and multi-episode bipolar affective disorders. Journal of Affective Disorders, 93, 185–292.
O’Donnell, B. F., Vohs, J. L., Hetrick, W. P., Carroll, C. A., & Shekhar, A. (2004). Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology, 53, 45–55.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Ozer, S., Ulusahin, A., Batur, S., Kabakci, E., & Saka, M. C. (2002). Outcome measures of interepisode bipolar patients in a Turkish sample. Society Psychiatry and Psychiatric Epidemiology, 37, 31–37.
Paradiso, S., Lamberty, G. J., Garvey, M. J., & Robinson, R. G. (1997). Cognitive impairment in the euthymic phase of chronic unipolar depression. Journal of Nervous and Mental Disorders, 185, 748–754.
Pfefferbaum, A., Ford, J. M., White, P., & Roth, W. T. (1989). P3 in schizophrenia is affected by stimulus modality, requirements, medication status, and negative symptoms. Archives of General Psychiatry, 46, 1035–1044.
Phillips, M. L., Drevets, W. C., Rauch, S. L., & Lane, R. (2003). Neurobiology of emotion perception: II. Implications for major psychiatric disorders. Biological Psychiatry, 54, 515–528.
Pizzagalli, D., Lehmann, D., Hendrick, A., Regard, M., Pascual-Marqui, R. D., & Davidson, R. J. (2002). Affective judgments of faces modulate early activity (160 ms) within the fusiform gyri. Neuroimage, 16, 663–677.
Polich, J. (2003). Theoretical overview of P3a and P3b. In J. Polich (Ed.), Detection of change: Event-related potential and fMRI findings (pp. 83–98). Boston: Kluwer.
Polich, J., & Herbst, K. L. (2000). P300 as a clinical assay. International Journal of Psychophysiology, 38, 3–19.
Post, R. M., Denicoff, K. D., Leverich, G. S., Altshuler, L. L., Frye, M. A., Suppes, T. M., et al. (2003). Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. Journal of Clinical Psychiatry, 64, 680–690. (quiz pp. 738-739).
Potts, G. F., Dien, J., Hartry-Speiser, A. L., McDougal, L. M., & Tucker, D. M. (1998). Dense sensor array topography of the event-related potential to task-relevant auditory stimuli. Electroencephalography and Clinical Neurophysiology, 106, 444–456.
Potts, G. F., Liotti, M., Tucker, D. M., & Posner, M. I. (1996). Frontal and inferior temporal cortical activity in visual target detection: Evidence from high spatially sampled event-related potentials. Brain Topography, 9, 3–14.
Potts, G. F., Patel, S. H., & Azzam, P. M. (2004). Impact of instructed relevance on the visual ERP. International Journal of Psychophysiology, 52, 197–209.
Pritchard, W. S., Sokhadze, E. M., & Houlihan, M. (2004). Effects of nicotine and smoking on event-related potentials: Review. Nicotine & Tobacco Research, 6, 961–984.
Quraishi, S., & Frangou, S. (2002). Neuropsychology of bipolar disorder: A review. Journal of Affective Disorders, 72, 209–226.
Rogers, M. A., Kasai, K., Koji, M., Fukuda, R., Iwanami, A., Nakagome, K., et al. (2004). Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research, 50, 1–11.
Roschke, J., & Wagner, P. (2003). A confirmatory study on the mechanisms behind reduced P300 waves in depression. Neuropsychopharmacology, 28, S9–S12.
Rossignol, M., Philippot, P., Douilliez, C., Crommelinck, M., & Campanella, S. (2005). The perception of fearful and happy facial expression is modulated by anxiety: An event-related potential study. Neuroscience Letters, 377(2), 115–120.
Rotenberg, V. S. (2004). The peculiarity of the right-hemisphere function in depression: Solving the paradoxes. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28, 1–13.
Rubinsztein, J. S., Fletcher, P., Rogers, R., Ho, L. W., Aigbirhio, F., et al. (2001). Decision-making in mania: A PET study. Brain, 124, 2550–2563.
Rubinsztein, J. S., Michael, A., Paykel, E. S., & Sahakian, B. J. (2000). Cognitive impairment in remission in bipolar affective disorder. Psychology Medicine, 30, 1025–1036.
Rubinsztein, J. S., Michael, A., Underwood, B. R., Tempest, M., & Sahakian, B. J. (2006). Impaired cognition and decision-making in bipolar depression but no ‘affective bias’ evident. Psychological Medicine, 36, 629–639.
Rubinsztein, J. S., & Sahakian, B. J. (2002). Cognitive impairment in bipolar disorder. British Journal of Psychiatry, 181, 440–441.
Salisbury, D. F., Shenton, M. E., & McCarley, R. W. (1999). P300 topography differs in schizophrenia and manic psychosis. Biological Psychiatry, 45, 98–106.
Salisbury, D. F., Shenton, M. E., Sherwood, A. R., Fischer, I. A., Yurgelun-Todd, D. A., Tohen, M., et al. (1998). First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Archives of General Psychiatry, 55, 173–180.
Santosh, P. J., Malhotra, S., Raghunathan, M., & Mehra, Y. N. (1994). A study of P300 in melancholic depression—Correlation with psychotic features. Biological Psychiatry, 35, 474–479.
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., & Lang, P. J. (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37, 257–261.
Schutter, D. J., de Haan, E. H. F., & van Honk, J. (2004). Functionally dissociated aspects in anterior and posterior electrocortical processing of facial threat. International Journal of Psychophysiology, 53, 29–36.
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(suppl 20), 22–33.
Sokhadze, E., & El-Mallakh, R. (2006, May). Event-related potential study of cognitive and behavioral impairments in bipolar disorder. Poster presented at the 18th annual convention of the Association for Psychological Sciences, New York, NY.
Sokhadze, E., El-Mallakh, R., Singh, S., & Tasman, A. (2007, February). Psychophysiology of residual affective biases in bipolar disorder. Presented at 38th Annual meeting of AAPB, Monterey, CA.
Souza, V. B. N., Muir, W. J., Walker, M. T., Glabus, M. F., Roxborough, H. M., Sharp, C. W., et al. (1995). Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biological Psychiatry, 37, 300–310.
Streit, M., Ioannides, A. A., Liu, L., Wölwer, W., Dammers, J., Gross, J., et al. (1999). Neurophysiological correlates of the recognition of facial expressions of emotion as revealed by magnetoencephalography. Brain Research: Cognitive Brain Research, 7, 481–491.
Strik, W. K., Ruchsow, M., Abele, S., Fallgatter, A. J., & Mueller, T. J. (1998). Distinct neurophysiological mechanisms for manic and cycloid psychoses: Evidence from a P300 study on manic patients. Acta Psychiatrica Scandinavica, 989, 459–466.
Surguladze, S., Brammer, M. J., Keedwell, P., Giampietro, V., Young, A. W., Travis, M. J., et al. (2005). A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry, 57, 201–209.
Thompson, J. M., Gallagher, P., Hughes, J. H., Watson, S., Gray, J. M., Ferrier, I. N., et al. (2005). Neurocognitive impairment in euthymic patients with bipolar affective disorder. British Journal of Psychiatry, 186, 32–40.
Tohen, M., Zarate, C. A., Jr., Hennen, J., Khalsa, H. M., Strakowski, S. M., Gebre-Medhin, P., et al. (2003). The McLean-Harvard first-episode mania study: Prediction of recovery and first recurrence. American Journal of Psychiatry, 160, 2099–2107.
Umbrich, D., Javitt, D., Novak, G., Bates, J., Pollack, S., Lieberman, J., et al. (1998). Effects of clozapine on auditory event-related potentials in schizophrenia. Biological Psychiatry, 44, 716–725.
Van Gorp, W. G., Altshuler, L., Theberge, D. C., Wilkins, J., & Dixon, W. (1998). Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence: A preliminary study. Archives of General Psychiatry, 55, 41–46.
Van Honk, J., Tuiten, A., van den Hout, M., de Haan, E. H. F., & Stam, H. (2001). Attentional biases for angry faces: Relationships to trait anger and anxiety. Cognition Emotion, 15, 279–297.
Vuilleumier, P., & Pourtois, G. (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45, 174–194.
West, R. (2003). Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia, 41, 1122–1135.
White, D. A., Myerson, J., & Hale, S. (1997). How cognitive is psychomotor slowing in depression: Evidence from a meta-analysis. Aging, Neuropsychology Cognition, 4, 166–174.
Wijers, A. A., Mulder, G., Gunter, T. C., & Smid, H. G. O. M. (1996). Brain potential analysis of selective attention. In O. Neumann & A. F. Sanders (Eds.), Handbook of perception and action. Vol 3: Attention (pp. 333–387). Tullamore, Ireland: Academic Press.
Wilder-Willis, K., Sax, K. E., Rosenberg, H. L., Fleck, D. E., Shear, P. K., & Strakowski, S. M. (2001). Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disorder, 3, 58–62.
Young, R. C., Biggs, J. T., Ziegler, V. E., & Meyer, D. A. (1978). A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry, 133, 429–435.
Yurgelun-Todd, D. A., Gruber, S. A., Kanayama, G., Killgore, W. D., Baird, A. A., & Young, A. D. (2000). FMRI during affect discrimination in bipolar affective disorder. Bipolar Disorder, 2, 237–248.
Zubieta, J. K., Huguelet, P., O’Neil, R. L., & Giordani, B. J. (2001). Cognitive function in euthymic bipolar I disorder. Psychiatry Research, 102, 9–20.
Acknowledgments
This study was supported by the University of Louisville School of Medicine intramural grant to E. Sokhadze.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sokhadze, E.M., Tasman, A., Tamas, R. et al. Event-related Potential Study of the Effects of Emotional Facial Expressions on Task Performance in Euthymic Bipolar Patients. Appl Psychophysiol Biofeedback 36, 1–13 (2011). https://doi.org/10.1007/s10484-010-9140-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-010-9140-z