Abstract
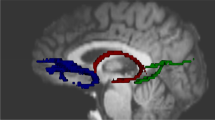
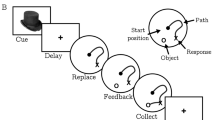
Numerous studies have shown that the hippocampus is critical for spatial memory. Within nonhuman research, a task often used to assess spatial memory is the radial arm maze. Because of the spatial nature of this task, this maze is often used to assess the function of the hippocampus. Our goal was to extrapolate this task to humans and examine whether healthy undergraduates utilize their hippocampus while performing a virtual reality version of the radial arm maze task. Thirteen undergraduates performed a virtual radial arm maze during functional magnetic resonance imaging. The brain maps of activity reveal bilateral hippocampal BOLD signal changes during the performance of this task. However, paradoxically, this BOLD signal change decreases during the spatial memory component of the task. Additionally, we note frontal cortex activity reflective of working memory circuits. These data reveal that, as predicted by the rodent literature, the hippocampus is involved in performing the virtual radial arm maze in humans. Hence, this virtual reality version may be used to assess the integrity of hippocampus so as to predict risk or severity in a variety of psychiatric disorders.
Similar content being viewed by others
References
Aguirre, G. K., Detre, J. A., et al. (1996). The parahippocampus subserves topographical learning in man. Cerebral Cortex, 6(6), 823–829.
Alvarado, M. C., & Rudy, J. W. (1995). Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behavioral Neuroscience, 109(2), 204–211.
Astur, R. S., St. Germain, S., Mathalon, D. H., D’Souza, D. C., Krystal, J. H., Constable, R. T., et al. (2004). Using virtual reality to investigate the functioning of the hippocampus in schizophrenia (Vol. 1, p. 62). Cybertherapy Abstracts.
Astur, R. S., Tropp, J., et al. (2004). Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behavioural Brain Research, 151(1–2), 103–115.
Baker, E. K., Demireva, P., et al. (2005). Virtual navigation in individuals with Alzheimer’s disease. New York: Cognitive Neuroscience Society.
Bingman, V. (1988). Unimpaired acquisition of spatial reference memory, but impaired homing performance in hippocampal ablated pigeons. Behavioral Brain Research, 27, 179–188.
Braak, H., & Braak, E. (1990). Cognitive impairment in Parkinson’s disease: Amyloid plaques, neurofibrillary tangles, and neuropil threads in the cerebral cortex. Journal of Neural Transmission Parkinsons Disease and Dementia Section, 2(1), 45–57.
Bremner, J. D., Randall, P., et al. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry, 41(1), 23–32.
Bunsey, M., & Eichenbaum, H. (1996). Conservation of hippocampal memory function in rats and humans. Nature, 379(6562), 255–257.
Cameron, K. A., Yashar, S., et al. (2001). Human hippocampal neurons predict how well word pairs will be remembered. Neuron, 30(1), 289–298.
Cohen, N. J., & Eichenbaum, H. (1993). Memory, amnesia, and the hippocampal system (Vol. XII, 330 pp). Cambridge, MA: MIT Press.
Ekstrom, A. D., Kahana, M. J., et al. (2003). Cellular networks underlying human spatial navigation. Nature, 425(6954), 184–187.
Freund, T. F., & Buzaki, G. (1996). Interneurons in the hippocampus. Hippocampus, 6, 347–470.
Frisk, V., & Milner, B. (1990). The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia, 28, 349–359.
Groen, G., Wunderlich, A. P., et al. (2000). Brain activation during human navigation: Gender-different neural networks as substrate of performance. Nature Neuroscience, 3(4), 404–408.
Hasselmo, M. E., & Wyble, B. P. (1997). Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behavioural Brain Research, 89(1–2), 1–34.
Huettel, S. A., McKeown, M. J., et al. (2004). Linking hemodynamic and electrophysiological measures of brain activity: Evidence from functional MRI and intracranial field potentials. Cerebral Cortex, 14(2), 165–173.
Jones-Gotman, M. (1986). Memory for designs: The hippocampal contribution. Neuropsychologia, 24(2), 193–203.
Jones-Gotman, M., Zatorre, R., et al. (1997). Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia, 35(7), 963–973.
Kahana, M. J., Sekuler, R., et al. (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature, 399(6738), 781–784.
Kandel, E. R., Schwartz, J. H., et al. (1995). Essentials of neural science and behavior. Stamford, UK: Appleton & Lange.
Kim, J. J., & Fanselow, M. S. (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677.
Lauritzen, M. (2001). Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism, 21(12), 1367–1383.
Logothetis, N. K., Pauls, J., et al. (2001). Neurophysiological investigation of the basis of the fMRI signal [see comment]. Nature, 412(6843), 150–157.
Maguire, E. A., Burgess, N., et al. (1998). Knowing where and getting there: A human navigation network. Science, 280(5365), 921–924.
Maguire, E. A., Frith, C. D., et al. (1998). Knowing where things are: Parahippocampal involvement in encoding object relations in virtual large-scale space. Journal of Cognitive Neuroscience, 10(1), 61–76.
Martin, A. (1999). Automatic activation of the medial temporal lobe during encoding: Lateralized influences of meaning and novelty. Hippocampus, 9(1), 62–70.
Milner, B. (1965). Memory disturbances after bilateral hippocampal lesions. In P. Milner & S. Glickman (Eds.), Cognitive processes and the brain. Princeton, NJ: D. Van Nostrand.
Montaldi, D., Mayes, A. R., et al. (1998). Associative encoding of pictures activates the medial temporal lobes. Human Brain Mapping, 6(2), 85–104.
Morris, R. G., Garrud, P., et al. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681–683.
Mumby, D. G., Astur, R. S., et al. (1999). Retrograde amnesia and selective damage to the hippocampal formation: Memory for places and object discriminations. Behavioral Brain Research, 106(1–2), 97–107.
O’Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34(1), 171–175.
O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon.
Olton, D., Becker, J., et al. (1979). Hippocampus, sapce, and memory. Behavioral and Brain Sciences, 2, 313–366.
Rudy, J. W., & Sutherland, R. J. (1995). Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus, 5(5), 375–389.
Sanchez-Arroyos, R., Gaztelu, J. M., et al. (1993). Hippocampal and entorhinal glucose metabolism in relation to cholinergic theta rhythm. Brain Research Bulletin, 32(2), 171–178.
Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neuropsychiatry and Clinical Neurosciences, 12(1), 103–113.
Sejnowski, T. J., Koch, C., et al. (1990). Computational neuroscience. In S. J. Hanson & C. R. Olson (Eds.), Connectionist modeling and brain function: The developing interface. Neural network modeling and connectionism (pp. 5–35). Cambridge, MA: MIT Press.
Shelton, A. L., & Gabrieli, J. D. E. (2004). Neural correlates of individual differences in spatial learning strategies. Neuropsychology, 18(3), 442–449.
Sherry, D. F., Jacobs, L. F., & Gualin, S. J. C. (1992). Spatial memory and adaptive specialization fo the hippocampus. Trends in Neurosciences, 15(8), 298–303.
Stern, C. E., & Hasselmo, M. E. (1999). Bridging the gap: Integrating cellular and functional magnetic resonance imaging studies of the hippocampus. Hippocampus, 9(1), 45–53.
St. Germain, S. A., Stevens, M., et al. (2004). Virtual navigation in patients with postraumatic stress disorder. San Francisco, CA: Society for Neuroscience.
Taylor, L. B. (1969). Localization of cerebral lesions by psychological testing. Clinical Neurosurgery, 16, 269–287.
Uecker, A., Barnes, C. A., et al. (1997). Hippocampal glycogen metabolism, EEG, and behavior. Behavioral Neuroscience, 111(2), 283–291.
Velakoulis, D., Stuart, G. W., et al. (2001). Selective bilateral hippocampal volume loss in chronic schizophrenia. Biological Psychiatry, 50(7), 531–539.
Waldvogel, D., van Gelderen, P., et al. (2000). The variability of serial fMRI data: Correlation between a visual and a motor task. Neuroreport: For Rapid Communication of Neuroscience Research, 11(17), 3843–3847.
Walker, J. A., & Olton, D. S. (1979). Spatial memory deficit following fimbria-fornix lesions: Independent of time for stimulus processing. Physiology and Behavior, 23(1), 11–15.
Wiebe, S. P., & Staeubli, U. V. (2001). Recognition memory correlates of hippocampal theta cells. Journal of Neuroscience, 21(11), 3955–3967.
Wood, E. R., Dudchenko, P. A., et al. (1999). The global record of memory in hippocampal neuronal activity [see comment]. Nature, 397(6720), 613–616.
Zola-Morgan, S., & Squire, L. R. (1990). The neuropsychology of memory: Parallel findings in humans and nonhuman primates. Annals of the New York Academy of Sciences, 608, 434–456.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Astur, R.S., St. Germain, S.A., Baker, E.K. et al. fMRI Hippocampal Activity During a VirtualRadial Arm Maze. Appl Psychophysiol Biofeedback 30, 307–317 (2005). https://doi.org/10.1007/s10484-005-6385-z
Issue Date:
DOI: https://doi.org/10.1007/s10484-005-6385-z