Abstract
Aspergillus ochraceus is a soil fungus known to produce ochratoxin A, a harmful secondary metabolite. Prevention and control of fungal pathogens mostly rely on chemical fungicides, which is one of the contributing factors in the emergence of the fungal resistance, hence novel methods for fungal eradication have been extensively researched. The cold atmospheric pressure (CAP) plasma generated in ambient air has been recently applied in microbial decontamination. Here we used the diffuse coplanar surface barrier discharge in inactivation of a toxigenic strain A. ochraceus. The plasma-treated conidia and mycelium exhibited morphological changes such as ruptures and desiccation. Mycelium dehydration and changes in the chemical composition of hyphal surface accompanied plasma treatment. The growth of 26 h old mycelia were significantly restricted after 30 s of plasma treatment. The conidial vitality declined 4 logs after 180 s of plasma exposure leading to almost complete decontamination. After shorter plasma treatment of conidia, the ochratoxin A (OTA) production increased at the early stage of cultivation, but the overall level was significantly reduced compared to untreated samples after longer cultivation. Our results indicated that the fungal growth and the OTA production were significantly changed by plasma treatment and underscored CAP plasma as a promising method in the decontamination of A. ochraceus without a risk to generate strains with increased OTA production.
Similar content being viewed by others
Introduction
The filamentous fungus, Aspergillus ochraceus is a frequent contaminant of economically important commodities, such as cereal grains, coffee beans and spices, during pre-harvest and storage. This contamination leads to enormous economic losses. Additionally, the soil fungus produces harmful secondary metabolites, such as ochratoxins with neurotoxic, genotoxic, hepatotoxic, teratogenic, and immunosuppressive effect (Wang et al. 2016).
Ochratoxin A (OTA) was first described in the A. ochraceus strain isolated on the Balkan Peninsula in 1965 (Van der Merwe et al. 1965). OTA is a white, odourless crystalline substance, thermostable with a melting point of approximately 168–173 °C. It is poorly soluble in water, but dissolves well in organic solvents (alcohols, ketones, and chloroform) (Tao et al. 2018). More than 20 ochratoxinogenic Aspergillus and Penicillium species are now known worldwide (Ismaiel and Papenbrock 2015; Gallo et al. 2017). OTA is the most toxic and prevalent toxin of the ochratoxin group and has been rated as a group 2B carcinogen by the International Agency for Research on Cancer and the World Health Organization. The maximum tolerable level is 5 µg kg− 1 OTA in cereal grains (in the EU) (WHO 1993). Although A. ochraceus is a well-known producer of OTA, other Aspergilli, such as A. westerdijkiae, produce an even higher amount of mycotoxin (Durand et al. 2018). Optimal OTA production was determined in water activity (aw) 0.98–0.99 (min aw 0.85) and at a temperature between 25 and 30 °C (Varga et al. 2015).
OTA toxicity is based on several mechanisms such as inhibition of protein synthesis, interference with phenylalanine metabolic systems, peroxidation of membrane lipids, impairment of calcium homeostasis, inhibition of mitochondrial respiration, and DNA damage (Ringot et al. 2006). OTA induces oxidative stress in cells, increases lipid peroxidation, causes protein damage and DNA breaks. On the other hand, antioxidants, especially glutathione, can protect cells from OTA-induced cytotoxicity and genotoxicity (Tao et al. 2018). Unlike aflatoxins, OTA contamination is significantly reduced during baking and food processing (Gupta et al. 2017).
Prevention and control of OTA are currently based on treatment with antifungal chemicals, such as benzimidazoles, aromatic hydrocarbons, or sterol biosynthesis inhibitors. However, the use of fungicides increases the risk of toxic residues in foods and could lead to fungal resistance (Hua et al. 2014). In contrast, cold atmospheric pressure (CAP) plasma represents an effective tool for microbial decontamination of different food surfaces without any traces of chemical contaminants (Cullen et al. 2017). The use of CAP plasma is also considered an innovative method in mycotoxin destruction (Hojnik et al. 2017). CAP plasma in ambient air at atmospheric pressure generates an electric field, UV radiation and biological reactive substances, such as charged particles and reactive molecules. The mode of plasma action studied on microorganisms can be explained by the synergistic effect of the reactive substances. A major role in the interaction of CAP plasma with cells was assigned to reactive oxygen species (ROS) (Ma et al. 2008; Arjunan et al. 2015). The increased level of the intracellular ROS in Aspergillus spp. can lead not only to oxidative stress with lethal consequences, but depending on the dose, the ROS can act as signalling molecules to regulate some physiological processes, such as production of secondary metabolites (Grintzalis et al. 2014).
Here we studied the effect of the CAP plasma generated by diffuse coplanar surface barrier discharge (DCSBD) in ambient air on spore and mycelium viability of the toxigenic fungus A. ochraceus. We determined all morphological and structural changes of the cell surface after plasma treatment and evaluated the CAP plasma treatment on OTA production in cultures inoculated by the plasma-treated conidia.
Materials and methods
Fungal strain and samples preparation
The toxigenic fungal strain of Aspergillus ochraceus Wilhelm CCM F-803 obtained from the Czech Collection of Microorganisms (Masaryk University, Czech Republic) was used. The conidia grown on Malt Extract Agar (Biolife, Italy) at 25 °C after 14 days were collected in sterile physiological saline solution with 0.1% Tween 80 (Biolife, Italy). The conidial suspension was filtered through three layers of sterile gauze and centrifuged at 12,000×g for 5 min. The number of spores was counted using a hemocytometer. The concentration of conidia was adjusted by sterile physiological saline solution on 1 × 109 conidia mL− 1.
The 20 µL of the conidia suspension was spread relatively even on a cellophane membrane placed on the surface of MEA agar plates. To determine the interactions of CAP plasma with conidia, the conidia spread on the cellophane membrane were treated immediately by plasma. To study the interactions of CAP plasma with fungal mycelium, conidia were germinated and grown at 27 °C for 26 h. Subsequently, the mycelium was treated with the CAP plasma. Three biological replicates were used in an experiment.
Plasma treatment
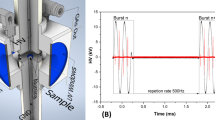
The plasma treatment of fungal cells was carried out using the DCSBD source of CAP plasma. Details of its characteristics and properties have been previously reported (Černák et al. 2009, 2011; Homola et al. 2012). The electrode system in the DCSBD consisted of a silver electrode system. The electrodes were embedded in 96% alumina. The thickness of the ceramic layer between the plasma and electrodes was 0.4 mm. The discharge was powered by a sinusoidal high frequency, high voltage signal (14 kHz, 20 kV). The DCSBD was generated at the input power 400 W. Under these conditions, the entire surface of the DCSBD ceramic plate was covered with a thin macroscopically diffuse plasma layer, and all samples were thus treated homogeneously. The sample temperature during plasma treatment did not exceed 35 °C. The experimental set-up is depicted in Fig. 1 (Šimončicová et al. 2018, modified). The fungal samples on the cellophane membrane were placed on the holder of the moving cart facing the ceramic plate of the DCSBD creating a 0.8 mm distance. In one cycle, each sample passed through the plasma field from one side to the other at a speed corresponding to the exposure time of 5 s. Longer treatments consisted of a number of cycles in order to achieve the desired treatment time.
Experimental set-up and schematic drawing of DCSBD electrode system (Šimončicová et al. 2018, modified)
Viability of conidia and mycelium
Plasma-treated conidia on a cellophane membrane were immersed in a sterile physiological saline solution with 0.01% Tween 80 (Biolife, Italy) and incubated on a rotary shaker (120 rpm) at 25 °C for 2 h. Diluted spore samples were placed on Malt Extract Agar and cultured at 25 °C for 48 h. The colony-forming units (CFU) were counted, and viability was calculated.
Plasma-treated mycelium was transferred into Malt Extract Broth (MEB; Biolife, Italy) with chloramphenicol (0.1 mg mL− 1; Thermo Fisher GmbH, Germany) and the mycelium was further cultured on a rotary shaker (120 rpm) at 25 °C for 24 h. After cultivation, the mycelium was collected by vacuum filtration, washed with 50 mL of distilled water, and dried at 80 °C to determine dry biomass weight.
Inactivation kinetics of conidia and mycelial cells
The inactivation kinetics of plasma effects on conidia was determined using the Weibull model (1) (Metselaar et al. 2013)
where \({\text{log}}_{10}{N}_{t}\) is the decadic logarithm of the number of viable conidia on cellophane after exposure to plasma for time \(t\); \({\text{log}}_{10}{N}_{0}\) is the decimal logarithm of the number of conidia on cellophane without plasma treatment; \(\Delta\) is the decimal reduction number; \({t}_{\Delta D}\) is the time needed to devitalise conidia by the order of ∆, and \(\beta\) is the parameter determining the shape of the curve. From the Eq. (1), the plasma exposure times necessary for devitalisation of conidia by 1–4 logs were determined.
The Hill model was used to determine the kinetics of mycelial inactivation. After plasma treatment, the percentage loss of dry biomass was determined relative to the untreated sample according to the Eq. (2)
where \({m}_{0}\) is the dry biomass weight of untreated mycelium after cultivation and \({m}_{t}\) is the dry biomass mass of mycelium treated after plasma treatment. The dependence of mycelial devitalisation on the plasma exposure duration was graphically determined using the Hill model (3) (Veroli et al. 2015)
in which \({E}_{Hill}\) is the effect achieved at a given time \(t\), \({E}_{\propto }\) is the maximum effect, \({E}_{0}\) is the plasma-free effect, \(H\) is the Hill exponent and \({ED}_{50}\) is the plasma dose (exposure duration) at which 50% is the obtained devitalising effect. Based on Eq. (3), we were able to determine the length of plasma exposure required to inactivate 50 and 90% of the mycelium grown on cellophane.
Scanning electron microscopy observation
The changes in the surface of A. ochraceus conidia and hyphae before and after plasma treatment were visualised by Scanning Electron Microscopy (SEM) using Vega II SBH (Tescan, Czech Republic). Samples for SEM measurements were coated with gold by the SEM Coating System (BIO-RAD E52-5199, Watford, England) at a pressure of 8 Pa in argon. The thickness of the gold layer was approximately 20 nm.
Fourier transform infrared spectroscopic analysis
Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) was used for detection of the changes in specific chemical groups on the surface of A. ochraceus mycelium before and after plasma treatment. FTIR spectra were obtained using a Bruker Vector 22 FTIR spectrometer with additional accessories, Pike MIRacle™ (Bruker Optics Inc. Billerica, MA, USA) in the range of 4000–500 cm− 1 during 20 scans, with 4 cm− 1 resolution using diamond crystal.
Ochratoxin A determination
Both untreated and 60 and 90 s plasma-treated conidia were cultivated in Petri dishes with Yeast Sucrose Agar consisting of 15% sucrose (Mikrochem, Slovakia), 2% yeast extract (Biolife, Italy) and 2% agar (Biolife, Italy) on a cellophane membrane. After 4 and 7 days of cultivation in the dark at 27 °C, we were able to determine the amount of OTA formed.
The biomass with cellophane was removed and 4 or 5 pieces of 2 g of agar plug (1 cm) were retrieved from the agar for OTA quantification. To determine OTA concentration in a culture medium, the commercial kit the RIDASCREEN® Ochratoxin A 30/15 (R-Biopharm AG, Germany) based on competitive enzyme immunoassay was used. Extraction and determination were performed according to the manufacturer´s instructions. The levels of OTA were determined in µg per g of growth medium and the number of CFU on Petri dishes.
Statistical analysis
The numerical data was expressed as a mean ± standard deviation (SD) for at least three replicated measurements. Significant differences between treatments were evaluated using one-way analysis of variance. Two groups of data were compared statistically by Student's t-test. The differences were considered significant for the P value < 0.05.
Results
The effect of plasma treatment on the viability of conidia and mycelium of A. ochraceus
The effect of CAP plasma on conidia viability at different time of exposure was determined as CFU counts. The decadic logarithm of the number of CFU of the plasma-untreated control was 6.73 ± 0.15. A statistically significant decrease in the log of the plasma-treated conidia was determined after 30-s long exposure. After 180 s of plasma treatment, the conidia viability decreased more than 4 logs (Table 1).
The dependence of the number of viable conidia on the length of plasma treatment time was determined using the Weibull model (1) which is shown in Fig. 2a. Using the Weibull Eq. (1), the parameters for each decimal reduction were calculated and the slope of the curve was determined (Table 2). According to the above equation, a decrease in the vitality of conidia, e.g. 3 logs was obtained after 46-s plasma treatment.
The effect of plasma inactivation of A. ochraceus hyphae at a different exposure times was determined as a percentage reduction in the ability of the treated mycelium to resume the growth. A statistically significant effect of CAP plasma on mycelial vitality (biomass growth) was determined after 5-s exposure (Table 1), when the average dry biomass weight loss was approximately 53% compared to the control (0%). After 15 s of treatment, a further significant decrease in vitality was observed.
To determine the ED50 and ED90, we used the Hill Eq. (3) to calculate the dependence of biomass growth on the duration of plasma exposure (Fig. 2b). Based on the Hill equation, a 50% decrease in the vitality of the mycelium on the cellophane membrane occurred at approximately 3 s of treatment with CAP plasma. A 90% decrease in vitality would be achieved at 28 s of treatment time (Table 2).
Surface changes in A. ochraceus conidia and mycelium
Changes in the cell wall surface of hyphae and conidia after 60 and 180 s of plasma treatment were observed by SEM (Fig. 3). The control sample (Fig. 3a) appeared to be decompressed, but intact with a smooth, tubular structure. Plasma-treated hyphae (Fig. 3b, c) differ from untreated ones by different depressions on the hyphae, and by separation of the thin transparent cover layer present on the surface. A different depression was detected also in the conidia and additionally, rupture of conidia was observed after 60 and 180 s of plasma treatment (Fig. 3e, f).
Changes in the chemical bond composition on the surface of A. ochraceus mycelium after plasma treatment were analysed by ATR-FTIR (Fig. 4). The decline in the signal intensity in the area between 3400 and 3100 cm− 1, which represent OH and NH stretching vibrations, is likely caused by dehydration after plasma treatment and possibly also by changes in the acetamido-group in chitin in the cell wall. The peak formation at 3280 cm− 1 and 3350 cm− 1 occurred after plasma treatment. Peaks in the region between 2800 and 3000 cm− 1 were attributed to the asymmetric and symmetric stretching vibration of C–H of the methylene group in fatty acid, respectively. The enhancement of signal intensities in the peaks was determined in the plasma-treated cells in the region from 950 to 1700 cm− 1. This region includes the spectra corresponding to C–O, aromatic –CH, C=O and –CN stretching vibration, NH deformation and bending, as well as the SO3 group. The increase in the intensity of the stretching vibrations of C=O and C–O groups on the plasma-treated samples could represent oxidation of biomolecules, such as lipids and polysaccharides.
Ochratoxin A production after cold plasma treatment
The differences in OTA production by 4 and 7-day old cultures grown from plasma-treated A. ochraceus conidia are shown in Table 3. The concentration of OTA in the medium calculated per number of CFUs after 4-day cultivation reached 13–75 µg 103 g−1CFU− 1. The conidia treated for 90 s after 4-day cultivation exhibited a 5-fold increase in OTA production compared to OTA produced by the untreated conidia.
After 7 days, the highest OTA level was determined in cultures grown from untreated conidia. Mycelium that grew from 60 and 90-s plasma-treated conidia however displayed an approximately 3-fold reduction of OTA than mycelium generated by untreated conidia.
Discussion
The effect of plasma treatment on the viability of conidia and mycelia of A. ochraceus
The effect of CAP plasma depends on the type of organism, cell, or tissue (Laroussi 2005; Mai-Prochnow et al. 2016; Bourke et al. 2017). CAP plasma exhibits inactivating effects against a wide range of microorganisms, including antibiotic resistant bacteria, bacterial endospores, bacterial and yeast biofilms, and filamentous fungi. Aspergillus spp. conidia are more resistant to plasma devitalisation than bacterial strains (Muranyi et al. 2007). Most studies on CAP plasma inactivation of filamentous fungi have focused on the treatment of conidia (Dasan et al. 2015, 2016). In many studies, various substrates, most commonly food or seeds, are usually covered by conidia and subsequently treated by plasma where the CFU counts per g substrate are determined (Suhem et al. 2013; Ouf et al. 2015; Yong et al. 2017). The carrier or matrix, as well as its morphology and surface structure on which conidia are deposited, play an important role in decontamination. In our study, we selected cellophane as a non-biological carrier since it exhibits different surface properties as compared to plant grains. The advantage of using cellophane in contrast to spherical carriers (grains) lies in the easy execution of the uniformity and homogeneity of plasma treatment of a thin cellophane layer containing conidia. To achieve homogeneity in the treatment of spherical carriers, it was necessary to implement a uniform rotation of the samples. Some matrices possess rough or porous surfaces where microbes could hide, which leads to a weaker inactivating effect by the plasma (Zahoranová et al. 2016, 2018). The wet cellophane itself forms a smooth layer without any crevices. However, prolonged plasma treatment of cellophane led to liquid evaporation of the cellophane carrier, which caused the cellophane to break easily. The longest treatment used in the study did not exceed 3 min. The short exposure of 3 min was sufficient to reduce the viability of A. ochraceus conidia by more than 4 logs (Tables 1 and 2).
Filamentous fungi can grow and colonize, forming substrate and aerial hyphae, fructification structures, conidia and ascospores, and others (Mcintyre et al. 2001; Bistis et al. 2003). Taking into consideration the differences in physiology of these cell forms, which had been formed by the same fungus, we believe that distinct cell types were affected differently when exposed to plasma treatment. The data demonstrated that vegetative hyphae were more sensitive than conidia (Table 1). In contrast to conidia, which are dormant cells used in asexual reproduction, hyphae are metabolically active multicellular forms. For these reasons, we expected that plasma-mediated inactivation of mycelium would be much quicker than that of conidia. The mycelium showed a 73% decrease in dry biomass weight after 30 s. Given the decrease in biomass growth capacity in the treated mycelium, we assumed that the respective biomass percentage had been devitalised (Table 1). Using the same plasma parameters and the same initial conidia concentration, the ED90/D value was higher for the mycelium (Table 2). Weibull distribution applied to model the inactivation of conidia in the first seconds of treatment may be slightly biased as indicated by β < 1 (early-life failures). The decrease in conidia vitality after 30 s may not have such a steep course over the first few seconds. Therefore, it would be reasonable to consider the values obtained after a longer exposure time than 30 s, from which it is apparent that conidia decreased by 3.5 log CFU from the original 6.73 log CFU.
Surface changes in A. ochraceus conidia and mycelia
Morphological changes after CAP plasma treatment were observed previously on the surface of cells of A. flavus, A. niger, and A. parasiticus. After plasma treatment, conidia aggregation, phialide destruction, cell shape changes, disruption of conidia sheaths, including cracks and pore-like structures on the conidial surface occurred (Dasan et al. 2015, 2016; Yoshino et al. 2013). Here, the microscopy of plasma-treated conidia of A. ochraceus was not as destructive even after 180 s of treatment (Fig. 3). Some conidia in A. ochraceus demonstrated ruptures, as well as the formation of pore-like structures and reduction in size due to dehydration. The morphology of most conidia seemed unaffected by plasma treatment. Nonetheless, conidia viability after plasma exposure of 180 s dropped 4 logs. We assume that the devitalising effect of DCSBD on A. ochraceus conidia could be linked to as of yet unspecified intracellular damages. DCSBD is generally considered to be a very environmentally friendly type of plasma, and surface changes were not observed in other biological matrices (Zahoranová et al. 2018).
Changes in the representation of functional groups of cellular coat layers were confirmed by ATR-FTIR analysis. Based on ATR-FTIR spectra we could confirm dehydration, which was shown as a decrease in the region for the vibration of OH groups (Fig. 4). We also observed changes in the region of the spectrum typical for glycosidic bonds. Here, we can assume that the polysaccharides present in the cell wall may have been oxidized. We also observed changes for the amide I and II regions. By increasing the intensity of the peaks for the vibrations of the C=O and C–O groups (Fig. 4), we believe that various types of macromolecules had been oxidized on the cell surface and possibly just below the surface.
Ochratoxin A production after cold plasma treatment
Mycotoxins, which are among the most dangerous natural substances, poison important crops, and products. To avoid the undesirable effects of mycotoxins, their occurrence is strictly controlled, and the subsequent disposal of degraded crops and products has a significant impact on the economy. In addition to their high toxicity, mycotoxins are highly stable to physical, chemical, or biological detoxifications (Durek et al. 2018). Therefore, it is particularly important to prevent contamination, especially by eliminating the fungal infection.
At present, there is a great interest in the degradation of these toxic products by plasma, but for now, we have not found any information about the effect of CAP plasma on mycotoxin production. So far, we have mainly encountered studies in which plasma-induced degradation of mycotoxin was observed. However, it is important to note that some of the cells were killed by plasma, and therefore the reduction of mycotoxins could be contributed to a decrease in viable fungal cells (Ouf et al. 2015; Sakudo et al. 2017; Shi et al. 2017). In our study, one of the goals was to investigate whether surviving fungal cells maintained the production of toxins, and if so, to determine whether the formation increased or decreased. One of the mechanisms of action of CAP plasma is based on oxidative stress. Coincidently, several studies have shown increased production of mycotoxins in response to the oxidative stress (Durek et al. 2018). Hence, we assumed that survivors of plasma treatment would be able to produce toxins at an increased rate. Indeed, the hypothesis of increased production of mycotoxins into the medium was confirmed by monitoring the production of ochratoxin A after 4-day cultivation, when there was an increase in production in the medium calculated per CFU after plasma treatment (Table 3). A significant increase in the amount of OTA on day 4 of the cultivation was seen after 90 s of plasma treatment. However, the results after 7 days of cultivation are interesting, since after 60 and 90 s exposure to plasma, the amount of OTA present was lower compared to the control. Plasma oxidative stress appeared to have only short-term effects, there was no increase in the amount of OTA detected in the culture medium after prolonged cultivation, however, the amount of these mycotoxins was significantly reduced. Based on these results, it could be concluded that after plasma treatment of conidia, mycotoxin production was initially stimulated in the early stages of mycelial growth, but OTA production was ultimately lower in the treated samples in comparison to those which were untreated. Durek et al. (2018) studied the changes in the amount of OTA on barley inoculated with Aspergillus niger or Penicillium verrucosum and subsequently treated for 1–3 min with DCSBD, which showed that despite the reduction in the number of surviving conidia, the amount of OTA increased under certain conditions. This was probably a consequence of the stress response of fungi to plasma treatment. However, it should be added that some of the results reported in the study were not statistically significant and the increase was measured only in the samples inoculated with mycelial suspension. In contrast, when inoculated with spore suspension, the amount of OTA in barley decreased. This was confirmed in an earlier study, where the reduction of OTA produced by A. niger grown on date palm fruits was also determined (Ouf et al. 2015). The use of CAP plasma remains a promising method in decontamination of A. ochraceus without any risk of generating strains with increased OTA production.
References
Arjunan KP, Sharma VK, Ptasinska S (2015) Effects of atmospheric pressure plasmas on isolated and cellular DNA—a review. Int J Mol Sci 16:2971–3016
Bistis GN, Perkins DD, Read ND (2003) Different cell types in Neurospora crassa. Fung Genet Rep 50:17–19
Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF (2017) Microbiological interactions with cold plasma. J Appl Microbiol 123:308–324
Černák M, Černáková L, Hudec I, Kováčik D, Zahoranová A (2009) Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur Phys J Appl Phys 47:22806
Černák M, Kováčik D, Ráheľ J, Sťahel P, Zahoranová A, Kubincová J, Tóth A, Černáková Ľ (2011) Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys Control Fusion 53:124031
Cullen PJ, Lalor J, Scally L, Boehm D, Milosavljević V, Bourke P, Keener K (2017) Translation of plasma technology from the lab to the food industry. Plasma Process Polym. https://doi.org/10.1002/ppap.201700085
Dasan BG, Mutlu M, Boyaci IH (2015) Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int J Food Microbiol 216:50–59
Dasan BG, Boyaci IH, Mutlu M (2016) Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control 70:1–8
Durand N, Fontana A, Meile JC, Suarez-Quiroz ML, Schorr-Galindo S, Montet D (2018) Differentiation and quantification of the ochratoxin A producers Aspergillus ochraceus and Aspergillus westerdijkiae using PCR-DGGE. J Basic Microbiol 59:158–165
Durek J, Schlüter O, Roscher A, Durek P, Fröhling A (2018) Inhibition or stimulation of ochratoxin a synthesis on inoculated barley triggered by diffuse coplanar surface barrier discharge plasma. Front Microbiol 9:1–9
Gallo A, Ferrara M, Perrone G (2017) Recent advances on the molecular aspects of ochratoxin A biosynthesis. Curr Opin Food Sci 17:49–56
Grintzalis K, Vernardis SI, Klapa MI, Georgiou CD (2014) Role of oxidative stress in sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Appl Environ Microbiol 80:55
Gupta RC, Lasher MA, Mukherjee IRM, Srivastava A, Lall R (2017) Aflatoxins, ochratoxins, and citrinin. In: Gupta RC (ed) Reproductive and developmental toxicology. Elsevier, Amsterdam, pp 945–962
Hojnik N, Cvelbar U, Tavčar-Kalcher G, Walsh JL, Križaj I (2017) Mycotoxin decontamination of food: cold atmospheric pressure plasma versus “classic” decontamination. Toxins (Basel) 9:1–19
Homola T, Matoušek J, Medvecká V, Zahoranová A, Kormunda M, Kováčik D, Černák M (2012) Atmospheric pressure diffuse plasma in ambient air for ITO surface cleaning. Appl Surf Sci 258:7135–7139
Hua H, Xing F, Selvaraj JN, Wang Y, Zhao Y, Zhou L, Liu X, Liu Y (2014) Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin a production. PLoS ONE 9:1–10
Ismaiel AA, Papenbrock J (2015) Mycotoxins: producing fungi and mechanisms of phytotoxicity. Agriculture 5:492–537
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391–400
Ma Y, Zhang GJ, Shi XM, Xu GM, Yang Y (2008) Chemical mechanisms of bacterial inactivation using dielectric barrier discharge plasma in atmospheric air. IEEE Trans Plasma Sci 36:1615–1620
Mai-Prochnow A, Clauson M, Hong J, Murphy AB (2016) Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep 6:1–11
Mcintyre M, Müller C, Dynesen J, Nielsen J (2001) Metabolic engineering. Adv Biochem Eng Biotechnol 73:103–128
Metselaar KI, den Besten HM, Abee T, Moezelaar R, Zwietering MH (2013) Isolation and quantification of highly acid resistant variants of Listeria monocytogenes. Int J Food Microbiol 666:508–514
Muranyi P, Wunderlich J, Heise M (2007) Sterilization efficiency of a cascaded dielectric barrier discharge. J Appl Microbiol 103:1535–1544
Ouf SA, Basher AH, Mohamed AAH (2015) Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J Sci Food Agric 95:3204–3210
Ringot D, Chango A, Schneider Y, Larondelle Y (2006) Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chemico 159:18–46
Sakudo A, Toyokawa Y, Misawa T, Imanishi Y (2017) Degradation and detoxification of aflatoxin B1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 73:619–626
Shi H, Ileleji K, Stroshine RL, Keener K, Jensen JL (2017) Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioprocess Technol 10:1042–1052
Šimončicová J, Kaliňáková B, Kováčik D, Medvecká V, Lakatoš B, Kryštofová S, Hoppanová L, Palušková V, Hudecová D, Ďurina P, Zahoranová A (2018) Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl Microbiol Biotechnol 102:6647–6658
Suhem K, Matan N, Nisoa M, Matan N (2013) Inhibition of Aspergillus flavus on agar media and brown rice cereal bars using cold atmospheric plasma treatment. Int J Food Microbiol 161:107–111
Tao Y, Xie S, Xu F, Liu A, Wang Y, Chen D, Pan Y, Huang L, Peng D, Wang X, Yuan Z (2018) Ochratoxin A: toxicity, oxidative stress and metabolism. Food Chem Toxicol 112:320–331
Van der Merwe KJ, Steyn PS, Fourie L, Scott DB, Theron JJ (1965) Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 205:1112
Varga J, Baranyi N, Chandrasekaran M, Vágvölgyi C (2015) Mycotoxin producers in the Aspergillus genus: an update. Acta Biol Szeged 59:151–167
Veroli GY, Di Fornari C, Goldlust I, Mills G, Koh SB, Bramhall JL, Richards FM, Jodrell DI (2015) An automated fitting procedure and software for dose-response curves with multiphasic features. Sci Rep 5:14701
Wang Y, Wang L, Liu F, Wang Q, Selvaraj JN, Xing F, Zhao Y, Liu Y (2016) Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins (Basel) 8:1–15
WHO—World Health Organization & International Agency for Research on Cancer (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. WHO 56
Yong IH, Lee H, Park S, Park J, Choe W, Jung S, Jo C (2017) Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat’s physicochemical properties. Meat Sci 123:151–156
Yoshino K, Matsumoto H, Iwasaki T, Kinoshita S, Noda K, Iwamori S (2013) Monitoring of sterilization in an oxygen plasma apparatus, employing a quartz crystal microbalance (QCM) method. Vacuum 93:84–89
Zahoranová A, Henselová M, Hudecová D, Kaliňáková B, Kováčik D, Medvecká V, Černák M (2016) Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem Plasma Process 36:397–414
Zahoranová A, Hoppanová L, Šimončicová J, Tučeková Z, Medvecká V, Hudecová D, Kaliňáková B, Kováčik D, Černák M (2018) Effect of cold atmospheric pressure plasma on maize seeds: enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem Plasma Process 38:969–988
Acknowledgements
This study was supported by the Slovak Research and Development Agency under the Contract No. APVV-16-0216.
Author information
Authors and Affiliations
Contributions
Conceptualization: JD, LH; Methodology: JD, LH, BK; Formal analysis and investigation: JD, PĎ, LH, DK, VM; Writing—original draft preparation: LH; Writing—review and editing: SK; Supervision: BK, AZ. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoppanová, L., Dylíková, J., Kováčik, D. et al. The effect of cold atmospheric pressure plasma on Aspergillus ochraceus and ochratoxin A production. Antonie van Leeuwenhoek 113, 1479–1488 (2020). https://doi.org/10.1007/s10482-020-01457-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-020-01457-8