Abstract
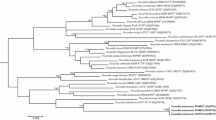
Three human clinical isolates (X1654, X1655, and W9944) were recovered from the sputum and bronchial washings of two patients with pulmonary infections. The 16S rRNA gene sequence analysis of the isolates showed that they share 100 % sequence similarity with each other and belong to the genus Nocardia. Close phylogenetic neighbours are Nocardia brevicatena ATCC 15333T (98.6 %) and Nocardia paucivorans ATCC BAA-278T (98.4 %). The in silico DNA–DNA relatedness between the isolates ranges from 96.8 to 100 % suggesting that they belong to the same genomic species. The DNA–DNA relatedness between X1654 and N. brevicatena ATCC 15333T is 13.3 ± 2.3 % and N. paucivorans ATCC BAA-278T is 18.95 ± 1.1 % suggesting that they do not belong to the same genomic species. Believed to represent a novel species, these isolates were further characterised to establish their taxonomic standing within the genus. Chemotaxonomic data for isolate X1654 are consistent with those described for the genus Nocardia: this isolate produced saturated and unsaturated fatty acids, tuberculostearic acid (15.9 %), the major menaquinone was MK-8 (H4cyclic), mycolic acid chain lengths ranged from 38 to 58 carbons, produced meso-diaminopimelic acid with arabinose, glucose, and galactose as the whole cell sugars. The polar lipids were diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylinositol mannosides. The DNA G+C content is 66.7 mol %. Based on the combination of phenotypic, chemotaxonomic, and genotypic data for X1654, X1655, and W9944, we conclude that these isolates represent a novel species within the genus Nocardia for which we propose the name Nocardia donostiensis sp. nov. with X1654T (=DSM 46814T = CECT 8839T) as the type strain.

Similar content being viewed by others
References
Auch AF, Von Jan M, Klenk H-P, Göker M (2010) Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2:117–134
Brown-Elliott B, Brown JM, Conville PS, Wallace RJ Jr (2006) Clinical and laboratory features of the Nocardia spp. Based on current molecular taxonomy. Clin Microbiol Rev 19:259–282
Cashion P, Hodler-Franklin MA, McCully J, Franklin M (1977) A rapid method for base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Clinical and Laboratory Standards Institute (2003) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA Clinical and Laboratory Standards Institute (2011) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standards M24-A2. Clinical Laboratory Standards Institute, Wayne, PA
Conville PS, Witebsky FG (2007) Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JHJ, Landry ML, Phaller MA (eds) Manual of clinical microbiology, 9th edn. American Society for Microbiology, Washington, DC, pp 515–542
Conville PS, Witebsky FG (2015) Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Jorgensen JH, Phaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (eds) Manual of clinical microbiology, 11th edn. American Society for Microbiology, Washington, DC, pp 504–535
Conville PS, Brown JM, Steigerwalt AG, Brown-Elliott BA, Witebsky FG (2008) Nocardia wallacei sp. nov., and Nocardiablaklockiae sp. nov., human pathogens and members of the “Nocardia transvalensis complex”. J Clin Microbiol 46:1178–1184
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Felsenstein J (1981) Evolutionary trees from DNA sequences: maximum-likelihood approach. J Mol Evol 17:368–376
Goodfellow M, Maldonado LA (2012) Genus 1. Nocardia Trevisan 1889AL. In: Goodfellow M, Kämpfer P, Busse H-J, Trujillo M, Suszuki K-I, Ludwig W, Whitman WB (eds) Bergey’s manual of systematic bacteriology. Springer, New York, pp 376–419
Goris J et al (2007) DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57(Pt 1):81–91
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Kim M, Hyun-Seok O, Sang-Cheol P, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351
Klatte S, Kroppenstedt RM, Rainey FA (1994) Rhodococcus opacus sp. nov., an unusual nutritionally versatile Rhodococcus species. Syst Appl Microbiol 17:355–360
Kroppenstedt RM (1982) Separation of bacterial menaquinones by HPLC using reverse phase (RP18) and a silver loaded ion exchanger as stationary phases. J Liq Chromatogr 5:2359–2367
Kroppenstedt RM (1985) Fatty acid and menaquinone analysis of actinomycetes and related organisms. In: Goodfellow M, Minnikin DE (eds) Chemical methods in bacterial systematics., SAB technical seriesAcademic Press, London, pp 173–199
Lasker BA, Moser BD, Brown JM (2011) Gordonia. In: Liu D (ed) molecular detection of human bacterial pathogens. CRC Taylor & Francis Group, Boca Raton, pp 95–110
Lauer AC, Nicholson AC, Humrighouse B, Emery B, Drobish A, Loparev V, McQuiston JR (2015) Genome sequences of Oblitimonas alkaliphila gen. nov. sp. nov. a novel bacterium of the Pseudomonadaceae family. Genome Announc. doi:10.1128/genomeA.01474-15
Lechevalier MP, Lechevalier HA (1970) Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol 20:435–443
Lechevalier MP, De Bievre C, Lechevalier H (1977) Chemotaxonomy of the aerobic actinomycetes: phospholipid composition. Biochem Syst Ecol 5:249–260
Meier-Kolthoff JP, AF, Klenk H-P, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform 14:60
Minnikin DE, O’Donnell AG, Goodfellow G, Alderson G, Athalye M, Schaal A, Parlett JH (1984) An intergrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. USFCC News 20:16
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bact 44:846–849
Staneck JL, Roberts GD (1974) Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol 28:226–231
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tindall BJ, Rosselló-Móra R, Busse H-J, Ludwig W, Kämpfer P (2010) Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60:249–266
Welsh O, Vera-Cabrera L, Salinas-Carmona MC (2013) Current treatment for Nocardia infections. Expert Opin Pharmacother 14:2387–2398
Weyant RS, Moss CW, Weaver RE, Hollis DG, Jordan JG, Cook EC, Daneshvar MI (1996) Identification of unusual pathogenic gram-negative and facultatively anaerobic bacteria, 2nd edn. Williams and Wilkins, Baltimore
Yassin AF, Rainey FA, Brzezinka H, Burghardt J, Lee HJ, Schaal KP (1995) Tsukamurella inchonensis sp. nov. Int J Syst Bacteriol 45:522–527
Zhi X-Y, Li W-J, Stakebrandt E (2009) An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of existing higher taxa. Int J Syst Evol Microbiol 59:589–608
Acknowledgments
To DSMZ for the wet -lab DDHS and Julian Larruskain MD, for his invaluable earlier work on Nocardia in our Microbiology Department at University Hospital Donostia.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed by authors contributing to this journal do not neccesarily reflect the opinions of the U.S department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors´ affiliated institutions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ercibengoa, M., Bell, M., Marimón, J.M. et al. Nocardia donostiensis sp. nov., isolated from human respiratory specimens. Antonie van Leeuwenhoek 109, 653–660 (2016). https://doi.org/10.1007/s10482-016-0667-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0667-8