Abstract
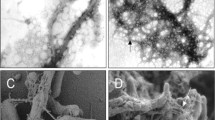
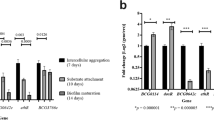
Organized bacterial communities, or biofilms, provide an important reservoir for persistent cells that are inaccessible or tolerant to antibiotics. Curli pili are cell-surface structures produced by certain bacteria and have been implicated in biofilm formation in these species. In order to determine whether these structures, which were suggested to be encoded by the Rv3312A (mtp) gene, have a similar role in Mycobacterium tuberculosis, we generated a Δmtp mutant and a mtp-complemented strain of a clinical isolate of M. tuberculosis and analyzed these strains for their ability to produce pili in comparison to the wild-type strain. Phenotypic analysis by transmission electron microscopy proved the essentiality of mtp for piliation in M. tuberculosis. We then compared biofilm formation of the derived strains in detergent-free Sauton’s media. Biofilm mass was quantified spectrophotometrically using crystal violet. Furthermore, we examined mtp gene expression by quantitative real-time PCR in wild-type cells grown under biofilm versus planktonic growth conditions. We found a 68.4 % reduction in biofilm mass in the mutant compared to the wild-type strain (P = 0.002). Complementation of the mutant resulted in a restoration of the wild-type biofilm phenotype (P = 0.022). We, however, found no significant difference between mtp expression in cells of the biofilm to those growing planktonically. Our findings highlight a crucial, but non-specific, role of pili in the biofilm lifestyle of M. tuberculosis and indicate that they may represent an important target for the development of therapeutics to attenuate biofilm formation, thereby potentially reducing persistence.




Similar content being viewed by others
References
Alteri CJ (2005) Novel pili of Mycobacterium tuberculosis. Ph.D. Thesis, The University of Arizona
Alteri CJ, Xicohténcatl-Cortes J, Hess S, Caballero-Olín G, Girón JA, Friedman RL (2007) Mycobacterium tuberculosis produces pili during human infection. Proc Natl Acad Sci USA 104:5145–5150
Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K (1989) Current protocols in molecular biology. Greene Publishing and Wiley Interscience, New York
Bardarov S, Bardarov S Jr, Pavelka MS Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR Jr (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007–3017
Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60:131–147
Canetti G (1955) Tubercle Bacillus in the pulmonary lesion of man: histobacteriology and its bearing on the therapy of pulmonary tuberculosis. Springer, New York
Carter G, Wu M, Drummond DC, Bermudez LE (2003) Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol 52:747–752
Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Åberg V, Walker JN, Seed PC, Almqvist F, Chapman MR, Hultgren SJ (2009) Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol 5:913–919
Collinson SK, Clouthier SC, Doran JL, Banser PA, Kay WW (1996) Salmonella enteritidis agfBAC operon encoding thin, aggregative fimbriae. J Bacteriol 178:662–667
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Gao Q, Kripke K, Arinc Z, Voskuil M, Small P (2004) Comparative expression studies of a complex phenotype: cord formation in Mycobacterium tuberculosis. Tuberculosis 84:188–196
Gerstel U, Rőmling U (2001) Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ Microbiol 3:638–648
Hammar M, Arnqvist A, Bian Z, Olsén A, Normark S (1995) Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol 18:661–670
Islam MS, Richards JP, Ojha AK (2012) Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms. Expert Rev Anti Infect Ther 10:1055–1066
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Larsen MH, Biermann K, Tandberg S, Hsu T, Jacobs WR Jr (2007) Genetic manipulation of Mycobacterium tuberculosis. Curr Protoc Microbiol 6:10A.2
Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orme IM, Basaraba RJ (2007) Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother 51:3338–3345
Lew JM, Kapopoulou A, Jones LM, Cole ST (2011) TubercuList- 10 years after. Tuberculosis (Edinb) 91:1–7
Marsollier L, Aubry J, Coutanceau E, André JP, Small PL, Milon G, Legras P, Guadagnini S, Carbonnelle B, Cole ST (2005) Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol 7:935–943
Middlebrook G, Dubos RJ, Pierce C (1947) Virulence and morphological characteristics of mammalian tubercle bacilli. J Exp Med 86:175–184
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Ojha AK, Hatfull GF (2012) Biofilms of Mycobacterium tuberculosis: new perspectives of an old pathogen. In: Cardona P (ed) Understanding tuberculosis—deciphering the secret life of the bacilli. Intech Open Access Publisher, Reijek, pp 181–192
Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR Jr, Hatfull GF (2008) Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol 69:164–174
Pang JM, Layre E, Sweet L, Sherrid A, Moody DB, Ojha A, Sherman DR (2012) The polyketide Pks1 contributes to biofilm formation in Mycobacterium tuberculosis. J Bacteriol 194:715–721
Paterson GK, Mitchell TJ (2004) The biology of Gram-positive sortase enzymes. Trends Microbiol 12:89–95
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Rost B, Yachdav G, Liu J (2004) The PredictProtein Server. Nucleic Acids Res 32(Web Server issue):W321–W326
Sasindran SJ, Torrelles JB (2011) Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front Microbiol 2:2
Spigelman M, Ma Z (2004) Mycobacterium tuberculosis: new tricks for an old bug. Expert Rev Anti Infect Ther 2:467–469
Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs WR Jr, Bloom BR (1991) New use of BCG for recombinant vaccines. Nature 351:456–460
van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD (1991) Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol 29:2578–2586
Velayati AA, Farnia P, Masjedi MR (2012) Pili in totally drug resistant Mycobacterium tuberculosis (TDR-TB). Int J Myco 1:57–58
World Health Organization (2012) Global tuberculosis report 2012. http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf. Accessed 6 March 2013
Acknowledgments
We thank Mr Mhlengi Vella Ncube (UKZN) for his contribution to the generation of the Δmtp mutant; Ms Charissa Naidoo (UKZN) for help with the statistical analysis; and the National Research Foundation (NRF), SA, Medical Research Council (MRC), SA, and College of Health Sciences (CHS), UKZN, for financial support. Mr S. Ramsugit gratefully acknowledges scholarship from the NRF and Canon Collins Trust.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramsugit, S., Guma, S., Pillay, B. et al. Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis . Antonie van Leeuwenhoek 104, 725–735 (2013). https://doi.org/10.1007/s10482-013-9981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-013-9981-6