Abstract
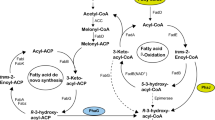
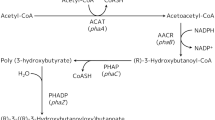
Four (R)-specific enoyl CoA hydratases (PhaJ) interconnect the β-oxidation pathway with PHA biosynthesis in Pseudomonas aeruginosa. The use of antisense technique and over-expression to delineate the role of two of these enzymes, PhaJ1 and PhaJ4 forms the basis of this study. It has been observed that P. aeruginosa recombinant with phaJ1 antisense construct, fed with different fatty acids, produces PHA with less hydroxy octanoate (7–11% reduction) and a proportionate increase in other monomer fractions, compared to that of the control. Recombinants bearing phaJ4 antisense construct are found to contain less hydroxy decanoate (10–11% reduction) and more or less equal amount of hydroxy octanoate, compared to that of the control. P. aeruginosa has produced PHA with more hydroxy octanoate and decanoate (6–17% increase), respectively, when PhaJ1 and PhaJ4 have been over-expressed individually or along with PhaC1. PhaJ1 and PhaJ4 are found to be involved mainly in the production of hydroxy octanoyl CoA and hydroxy decanoyl CoA, respectively, in P. aeruginosa. The strongest accumulation of hydroxy octanoate and hydroxy decanoate has been observed along with hydroxy butyrate, in PHA, produced by E. coli, when PhaC1 has been co-expressed with PhaJ1 and PhaJ4, respectively. We have demonstrated, for the first time, the polymerization of hydroxy butyryl CoA monomers in recombinant E. coli by PhaC1 of P. aeruginosa.


Similar content being viewed by others
References
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly (β-hydroxyalkanoates) for potential application as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Chakrabarty AM, Mylorie JR, Friello DA, Vacca JG (1975) Transformation of Pseudomonas putida and Escherichia coli with plasmid-linked drug-resistance factor DNA. Proc Natl Acad Sci USA 72:3647–3651
Chen JY, Liu T, Zheng Z, Chen JC, Chen GQ (2004) Polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas stutzeri1317 had different substrate specificities. FEMS Microbiol Lett 234:231–237
Chen JY, Song G, Chen GQ (2006) A lower specificity PhaC2 synthase from Pseudomonas stutzeri catalyses the production of copolyesters consisting of short-chain-length and medium-chain-length 3-hydroxyalkanoates. Antonie van Leeuwenhoek 89:157–167
Choi JC, Shin HD, Lee YH (2003) Modulation of 3-Hydroxy valerate molar fraction in poly (3-hydroxybutyrate-3-hydroxyvalerate) using Ralstonia eutropha transformant co-amplifying phbC and NADPH generation-related zwf genes. Enzyme Microb Technol 32:178–185
Eggink G, de Waard P, Huijberts GNM (1992) The role of fatty acid biosynthesis and degradation in the supply of substrate for poly (3-hydroxy alkanoates) formation in Pseudomonas putida. FEMS Microbiol Rev 103:159–164
Fiedler S, Steinbuchel A, Rehm BHA (2002) The role of the fatty acid β- oxidation multienzyme complex from Pseudomonas oleovorans in poly-hydroxyalkanoate biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes phaJ from P. oleovorans and Pseudomonas putida. Arch Microbiol 178:149–60
Fukui T, Doi Y (1997) Cloning and analysis of the poly (3-hydroxybutyrate-co-3-hydroxy hexanoate) biosynthesis genes of Aeromonas caviae. J Bacteriol 179:4821–4830
Fukui T, Shiomi N, Doi Y (1998) Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J Bacteriol 180:667–673
Fukui T, Yokomizo S, Kobayashi G, Doi Y (1999) Co-expression of polyhydroxyalkanoate synthase and (R)-specific enoyl CoA hydratase genes of Aeromonas caviae establishes copolyester biosynthesis pathway in Escherichia coli. FEMS Microbiol Lett 17:69–75
Fukui T, Kichise T, Iwata T, Doi Y (2001) Characterization of 13 kDa granule associated protein in Aeromonas caviae and biosynthesis of polyhydroxyalkanoate with altered molar composition by recombinant bacteria. Biomacromolecules 2:148–153
Good L, Nielsen PE (1998) Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol 16:355–358
Han J, Qiu YZ, Liu DC, Chen GQ (2004) Engineered Aeromonas hydrophila for enhanced production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomer composition. FEMS Microbiol Lett 239:195–201
Ji Y, Marra A, Rosenberg M, Woodnutt G (1999) Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J Bacteriol 181:6585–6590
Ji Y, Zhang B, Van Horn SF, Warren P, Woodnutt G, Burnham MKR, Rosenberg M (2001) Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293:2266–2269
Klinke S, Ren Q, Witholt B, Kessler B (1999) Production of medium chain length poly(3-hydroxyalkanoates) from gluconate by recombinant Escherichia coli. Appl Environ Microbiol 65:540–548
Langenbach S, Rehm BHA, Steinbuchel A (1997) Functional expression of the PHA synthase gene, phaC1 from Pseudomonas aeruginosa in Escherichia coli results in poly(3-hydroxyalkanoate) synthesis. FEMS Microbiol Lett 150:303–309
Moskowitz GJ, Merrick JM (1969) Metabolism of poly-β-hydroxybutyrate. Enzymatic syntesis of D-(2)-β-hydroxybutyryl coenzyme A by an enoyl hydratase from Rhodospirillum rubrum. Biochemistry 8:2748–2755
Park SJ, Lee SY (2003) Identification and characterization of a new enoyl coenzyme A hydratase involved in biosynthesis of mcl-PHA in recombinant Escherichia coli. J Bacteriol 185:5391–5397
Park SJ, Lee SY (2004) New FadB homologous enzymes and their use in enhanced biosynthesis of medium-chain-length polyhydroxyalkanoates infadB mutant Escherichia coli. Biotechnol Bioeng 86:681–686
Park SJ, Park JP, Lee SY (2002) Metabolic engineering of Escherichia coli for the production of medium chain length polyhydroxyalkanoates rich in specific monomer. FEMS Microbiol Lett 214:217–222
Qi Q, Rehm BHA, Steinbuchel A (1997) Synthesis of poly (3-hydroxyalkanoates) in Escherichia coli expressing the PHA synthase gene phaC2 from Pseudomonas aeruginosa: comparison of PhaC1 and PhaC2. FEMS Microbiol Lett 157:155–162
Qi Q, Steinbuchel A, Rehm BHA (1998) Metabolic routing towards polyhydroxyalkanoic acid synthesis in recombinant Escherichia coli (fadR): inhibition of fatty acid β-oxidation by acrylic acid. FEMS Microbiol Lett 168:89–94
Rehm BHA, Steinbuchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 25:3–9
Rehm BHA, Kruger N, Steinbuchel A (1998) A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis: the phaG gene from Pseudomonas putida KT2440 encodes a 3-hydroxy acyl-ACP CoA transferase. J Biol Chem 273:24044–24051
Ren Q, Sierro N, Witholt B (2000) FabG, an NADPH-dependant 3-ketoacyl-ACP reductase of Pseudomonas aeruginosa, provides precursors for medium chain length poly-3- hydroxy alkanoate biosynthesis in Escherichia coli. J Bacteriol 182:2978–2981
Ren Q, van Beilen JB, Sierro N, Zinn M, Kessler B, Witholt B (2005) Expression of PHA polymerase genes of Pseudomonas putida in Escherichia coli and its effect on PHA formation. Antonie van Leeuwenhoek 87:91–100
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sato S, Nomura CT, Abe H, Doi Y, Tsuge T (2007) Poly [(R)-3-Hydroxybutyrate] formation in Escherichia coli from glucose through an enoyl-CoA hydratase-mediated pathway. J Biosci Bioeng 103:38–44
Snell KD, Feng F, Zhong L, Martin D, Madison LL (2002) YfcX enables Medium-chain-length poly (3-hydroxyalkanoate) formation from fatty acids in recombinant Escherichia coli fadB strains. J Bacteriol 184:5696–5705
Spector T (1978) Refinement of Coomassie Blue Method of protein quantitation. Anal Biochem 86:142–146
Timm A, Steinbuchel A (1990) Formation of polyesters consisting of medium chain length 3-hydroxy alkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent Pseudomonads. Appl Environ Microbiol 56:3360–3367
Timm A, Steinbuchel A (1992) Cloning and molecular analysis of the poly (3-hydroxy alkanoates) gene locus of Pseudomonas aeruginosa PA01. Eur J Biochem 209:15–30
Tsuge T, Fukui T, Matsusaki H, Taguchi S, Kobayashi G, Ishizaki A (2000) Molecular cloning of two (R)-specific enoyl CoA hydratases genes from Pseudomonas aeruginosa and their use for polyhydroxy alkanoates synthesis. FEMS Microbiol Lett 184:193–198
Tsuge T, Taguchi S, Taguchi K, Doi Y (2003) Molecular characterization and properties of (R)-specific enoyl CoA hydratases from Pseudomonas aeruginosa: Metabolic tools for synthesis of polyhydroxy alkanoates via fatty acid β-oxidation. Int J Biol Macromol 31:195–205
Williamson DH, Wilkinson JF (1958) The isolation and estimation of poly β- hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol 19:198–200
Acknowledgements
The authors wish to thank Department of Biotechnology, India, for funding the project. Reeta Davis gratefully acknowledges Council of Scientific and Industrial Research, India, for research fellowship. The LS1298Kan::fadB1 was kindly provided by Eliza McKinney of CLF Medical Technology Acceleration Program, Inc. New York, USA. We are grateful for Herbert P. Schweizer of Colorado State University for having provided the plasmid pBSP II KS (−) and the strain PA01.We thank Mr. P.S. Kulashekhar, C.F.T.R.I. for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, R., Chandrashekar, A. & Shamala, T.R. Role of (R)-specific enoyl coenzyme A hydratases of Pseudomonas sp in the production of polyhydroxyalkanoates. Antonie van Leeuwenhoek 93, 285–296 (2008). https://doi.org/10.1007/s10482-007-9203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-007-9203-1