Abstract
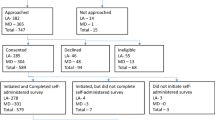
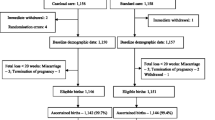
We evaluated the effect of an option B-plus Enhanced Adherence Package (BEAP), on early ART uptake in a randomized controlled trial. HIV-positive, ART naïve pregnant women in Lusaka, Zambia, were randomized to receive BEAP (phone calls/home visits, additional counseling, male partner engagement and missed-visit follow-up) versus standard of care (SOC). The primary outcome was initiating and remaining on ART at 30 days. Analysis was by intention to treat (ITT) using logistic regression. Additional per protocol analysis was done. We enrolled 454 women; 229 randomized to BEAP and 225 to SOC. Within 30 days of eligibility, 445 (98.2%) initiated ART. In ITT analysis, 82.5% BEAP versus 80.4% SOC participants reached primary outcome (crude relative risk [RR] 1.03; 95% confidence interval [CI] 0.91–1.16; Wald test statistic = 0.44; p-value = 0.66). In per protocol analysis, (92 participants (40.2%) excluded), 91.9% BEAP versus 80.4% SOC participants reached primary outcome (crude RR 1.14; 95% CI 1.02–1.29; Wald test statistic = 2.23; p-value = 0.03). Early ART initiation in pregnancy was nearly universal but there was early drop out suggesting need for additional adherence support.
This trial was registered at ClinicalTrials.gov (trials number NCT02459678) on May 14, 2015.

Similar content being viewed by others
References
WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approacg. 2nd ed. Geneva: WHO; 2016.
Chagomerana MB, Miller WC, Tang JH, Hoffman IF, Mthiko BC, Phulusa J, et al. Optimizing prevention of HIV mother to child transmission: duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS ONE. 2018;13(4):e0195033.
Myer L, Phillips TK, McIntyre JA, Hsiao NY, Petro G, Zerbe A, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town. S Afr HIV Med. 2017;18(2):80–8.
Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61(11):1715–25.
van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17(1):18994.
Kalua T, Barr BAT, Van Oosterhout JJ, Mbori-Ngacha D, Schouten EJ, Gupta S, et al. Lessons learned from option B+ in the evolution toward “test and start” from Malawi, Cameroon, and the United Republic of Tanzania. J Acquir Immune Defic Syndr. (1999). 2017;75(Suppl 1):S43.
Tenthani LHA, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28(4):589–98.
Ministry of Health Z. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection. Lusaka: Ministry of Health Z; 2014.
Tweya H, Gugsa S, Hosseinipour M, Speight C, Ng'ambi W, Bokosi M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in O ption B+ PMTCT programme in L ilongwe, M alawi. Trop Med Int Health. 2014;19(11):1360–6.
Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study. Lancet HIV. 2016;3(4):e175–e182182.
Dionne-Odom J, Massaro C, Jogerst KM, Li Z, Deschamps M-M, Destine CJ, et al. Retention in care among HIV-infected pregnant women in haiti with PMTCT option B. AIDS Res Treat. 2016. https://doi.org/10.1155/2016/6284290.
Chan AK, Kanike E, Bedell R, Mayuni I, Manyera R, Mlotha W, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc. 2016;19(1):20672.
Colvin CJ, Konopka S, Chalker JC, Jonas E, Albertini J, Amzel A, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(10):e108150.
Geldsetzer P, Yapa HMN, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19(1):20679.
Nance N, Pendo P, Masanja J, Ngilangwa DP, Webb K, Noronha R, et al. Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: A cluster-randomized trial. PLoS ONE. 2017;12(8):e0181919.
Rollins NC, Essajee SM, Bellare N, Doherty M, Hirnschall GO. Improving retention in care among pregnant women and mothers living with HIV: lessons from INSPIRE and implications for future WHO guidance and monitoring. J Acquir Immune Defic Syndr. (1999). 2017;75(2):S111.
Herce ME, Mtande T, Chimbwandira F, Mofolo I, Chingondole CK, Rosenberg NE, et al. Supporting option B+ scale up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: results from a serial cross-sectional study. BMC Infect Dis. 2015;15(1):328.
Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis. 2015;62(7):935–44.
Domercant JW, Puttkammer N, Young P, Yuhas K, François K, Grand Pierre R, et al. Attrition from antiretroviral treatment services among pregnant and non-pregnant patients following adoption of Option B+ in Haiti. Global Health Action. 2017;10(1):1330915.
Zürcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Tropical Med Int Health. 2017;22(4):375–87.
Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth. 2017;17(1):94.
Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(11):e111421.
Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3(5):e202–e211211.
Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. (1999). 2012;59(1):86.
Mitiku I, Arefayne M, Mesfin Y, Gizaw M. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. 2016;19(1):20662.
Yotebieng M, Thirumurthy H, Moracco KE, Kawende B, Chalachala JL, Wenzi LK, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV. 2016;3(2):e85–e93.
DiCarlo A, Fayorsey R, Syengo M, Chege D, Sirengo M, Reidy W, et al. Lay health worker experiences administering a multi-level combination intervention to improve PMTCT retention. BMC Health Serv Res. 2018;18(1):17.
Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health. 2018;23(2):136–48.
Fayorsey RN, Wang C, Chege D, Reidy W, Syengo M, Owino SO, et al. Effectiveness of a Lay Counselor-led combination intervention for retention of mothers and infants in HIV Care: a randomized trial in Kenya. J Acquir Immune Defic Syndr. (1999). 2019;80(1):56.
Acknowledgements
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreement number U01GH000530. Additional investigator support was provided by the National Institutes of Health (K24 AI120796). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies. The authors acknowledge the B-Plus study team for their work in participant recruitment and follow-up; and all the HIV infected pregnant women who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mubiana-Mbewe, M., Bosomprah, S., Kadota, J.L. et al. Effect of Enhanced Adherence Package on Early ART Uptake Among HIV-Positive Pregnant Women in Zambia: An Individual Randomized Controlled Trial. AIDS Behav 25, 992–1000 (2021). https://doi.org/10.1007/s10461-020-03060-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-020-03060-4