Abstract
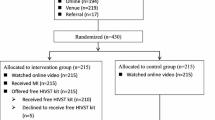
Systematic face-to-face pre-HIV test counseling is costly and may discourage clients to present for regular testing. In a randomized, controlled, non-inferiority trial conducted in four facilities providing free-of-charge anonymous HIV testing in Thailand, participants received either: standard counseling according to national guidelines (reference); computer-assisted counseling: interactive counseling on a tablet computer followed by an invitation to ask questions to the counselor; or on-demand counseling: invitation to ask questions to the counselor. Primary endpoint was a HIV retest within 7 months after enrolment visit. Following the planned interim analysis, on-demand counseling was discontinued for futility. In the final analysis in 1036 HIV-uninfected at-risk participants, computer-assisted counseling was non-inferior to standard counseling and had similar acceptability and improvements in HIV knowledge and sexual risk behaviors; however, it significantly reduced the time spent by counselors on counseling. Implementation of pre-HIV test computer-assisted counseling may ease the burden on staff involved in HIV testing.


Similar content being viewed by others
References
Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17 (quiz CE1-4).
DiNenno EA. Recommendations for HIV screening of gay, bisexual, and other men who have sex with men—United States, 2017. MMWR Morb Mortal Wkly Rep [Internet]. https://www.cdc.gov/mmwr/volumes/66/wr/mm6631a3.htm. (2017). Accessed Jan 23 2019.
World Health Organization. Consolidated guidelines on HIV testing services. Geneva: WHO; 2015.
UNAIDS. Joint United Nations Programme on HIV/AIDS. Ending AIDS: progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017.
Stephenson R, White D, Darbes L, Hoff C, Sullivan P. HIV testing behaviors and perceptions of risk of HIV infection among MSM with main partners. AIDS Behav. 2015;19(3):553–60.
Pham MD, Aung PP, Paing AK, Pasricha N, Agius PA, Tun W, et al. Factors associated with HIV testing among young men who have sex with men in Myanmar: a cross-sectional study. J Int AIDS Soc. 2017. https://doi.org/10.1002/jia2.25026.
Geter A, Herron AR, Sutton MY. HIV-related stigma by healthcare providers in the United States: A systematic review. AIDS Patient Care STDS. 2018;32(10):418–24.
Otten MW, Zaidi AA, Wroten JE, Witte JJ, Peterman TA. Changes in sexually transmitted disease rates after HIV testing and posttest counseling, Miami, 1988 to 1989. Am J Public Health. 1993;83(4):529–33.
Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med [Internet]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4596465/. (2015). Accessed Nov 8 2017.
Metsch LR, Feaster DJ, Gooden L, Schackman BR, Matheson T, Das M, et al. Effect of risk-reduction counseling with rapid HIV testing on risk of acquiring sexually transmitted infections: the AWARE randomized clinical trial. JAMA. 2013;310(16):1701–10.
Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Szekeres G, et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: a factorial randomised controlled trial. Lancet Global Health. 2013;1(3):e137–45.
Nöstlinger C, Platteau T, Bogner J, Buyze J, Dec-Pietrowska J, Dias S, et al. Implementation and operational research: computer-assisted intervention for safer sex in HIV-positive men having sex with men: findings of a european randomized multi-center trial. J Acquir Immune Defic Syndr. 2016;71(3):e63–72.
Fisher JD, Amico KR, Fisher WA, Cornman DH, Shuper PA, Trayling C, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav. 2011;15(8):1635–46.
Naar-King S, Outlaw AY, Sarr M, Parsons JT, Belzer M, Macdonell K, et al. Motivational Enhancement System for Adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38(6):638–48.
Kurth AE, Spielberg F, Cleland CM, Lambdin B, Bangsberg DR, Frick PA, et al. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: findings from a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;65(5):611–20.
Thailand Ministry of Public Health. Thailand National Guidelines on HIV/AIDS Treatment and Prevention [Internet]. http://www.thaiaidssociety.org/images/PDF/hiv_thai_guideline_2560.pdf. (2017). Accessed Apr 25, 2019.
Thailand Ministry of Public Health. Guideline on HIV Counseling & Testing for Same Day Result [Internet]. http://e-library-aidssti.ddc.moph.go.th/books/download/309. Accessed Apr 25, 2019.
Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15.
Carey MP, Schroder KEE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14(2):172–82.
Bratton DJ, Phillips PP, Parmar MK. A multi-arm multi-stage clinical trial design for binary outcomes with application to tuberculosis. BMC Med Res Methodol. 2013;13:139.
Wang C, Keller D, Lan K. Sample size re-estimation for binary data via conditional power. Proceedings of the American Statistical Association Biopharmaceutical Section. 2002;1:3621–6.
MiniMatters. The Best Video Length for Different Videos on YouTube [Internet]. https://www.minimatters.com/youtube-best-video-length/. Accessed Apr 25, 2019.
Thailand National Statistical Office. Key Findings: The 2009 Reproductive Health Survey [Internet]. http://web.nso.go.th/en/survey/reprod/data/rhs09_100810.pdf. Accessed Apr 25, 2019.
Acknowledgements
The Napneung project was supported by a grant from Expertise France through the 5% Initiative program (14SANIN204). We thank the members of the Advisory Committee: Sumet Ongwandee, Sombat Thanprasertsuk and Nakorn Premsri (Department of Diseases Control, Ministry of Public Health, Nonthaburi, Thailand), Suchada Chaivoot (National Health Security Office, Nonthaburi, Thailand), Mukta Sharma (World Health Organization, Thailand Country Office, Nonthaburi, Thailand), Chutima Jaruwat and Withun Wongthip (Provincial Public Health Office, Chiang Mai, Thailand), Eric Fleutelot (French Embassy to Thailand, Bangkok, Thailand), Pongthorn Chanlearn (M-Plus, Chiang Mai, Thailand), Ratchadet Reaukhamfu (Caremat, Chiang Mai, Thailand), Bram Press and Namisi Jate (MAP Foundation, Chiang Mai, Thailand), Boonnium Wongjaikham (Thai AIDS Treatment Action Group, Chiang Mai, Thailand), Thitipong Sangyoung (Thai Drug Users’ Network, Chiang Mai, Thailand), Monthira Metha (Fah Mai Clinic, Chiang Mai, Thailand) and Maleerat Vandriesten (Empower, Chiang Mai, Thailand). We also thank all Napneung partners, in particular: ACCESS, CAREMAT, EMPOWER, MAP Foundation, M-Plus, Namklong Colorful, PIMAN Center, PLAN International, Thai AIDS Treatment Action Group and Thai Drug Users' Network; Chiangrai Prachanukroh Hospital, Nakornping Hospital, Sanpatong Hospital, Sarapee Hospital and STIs Clinic of the Office of Disease Prevention and Control Region 1; Chiang Rai Rajabhat University and Mae Fah Luang University; Provincial Public Health Offices; and Department of Disease Control, Thai Ministry of Public Health. We also thank all members of the Napneung project team: Giuliana Zegarra, Paporn Mongkolwat, Prueksalada Khiaokham, Suphakrit Jitrarat and Wanlee Kongnim (project coordination); Ampika Kaewbundit, Areerat Kongphonoi, Chutharat Kasemrat, Duanpen Panyasak, Jiraporn Khamkon, Kanyanee Preechapongmit, Krisana Suphayos, Laddawan Laomanit, Nantawan Wangsaeng, Nilawan Chetacha, Ninutcha Paengsai, Panaddar Phutthakham, Paporn Mongkolwat, Phornphimon Moolnoi, Prapan Luangsook, Subenya Natthanaboon, Tanawan Samleerat, Thipsuda Krueyot, Tiwacha Thimakam, Wanlee Kongnim, Warunee Khamjakkaew, Warunee Srisuk and Woottichai Khamduang (testing and counseling); Boonnium Wongjaikham, Chalathon Jantarapanya, Chittiwa Phumsawai, Jamreon Teeravittayakul, Kanjana Saengkham, Malee Kaewprom, Monthira Metha, Nipa Chomphupa, Prapatsorn Chaisopa, Ratchadet Reaukhamfu, Rodjana Yeepua, Saidao Sanko, Sirintorn Juntong, Sompol Punyasriwichai, Supaluck Nammuang, Thitipong Sangyoung and Wipawan Wongsawasdi (PHPT Community Advisory Board in Chiang Mai); Akrkapong Preechapongmit, Anupab Jampatong, Anyamanee Khattiya, Apirak Jittarat, Areerat Kongponoi, Bancha Kumsiri, Boonrod Suwanpa, Chalita Sakrew, Chopet Nobpharat, Daoruang Sangchan, Daranrat Nuntasonbut, Dawaree Tongcheepcha, Dumrongdech Reunsung, Ekapan Chaiwangras, Jedsada Srijanta, Jinjutha Darasilp, Jirayut Sitasoy, Kajorndej Panichadul, Kajorndej Surapanichadul, Kamolpop Moungtanee, Kamonporn Preechapongmit, Kanok Fuslin, Kanyanee Kaewmamueng, Khwanchanok Saenkriang, Kitipong Daenpen, Kittikun Jantaracha, Kritsana Suphayos, Kulnaree Klomkaew, Kulthida Mueangchanta, Lukkana Sitiritkawin, Maliwan Budsuwan, Manthana Nunta, Mathurada Kanthalee, Metinee Khamwiang, Nattachai Kumket, Nattarika Prommatiam, Natthaporn Chinda, Nawakhun Prungrathanasen, Nonthacha Yodying, Nutnicha Parjarnthit, Paradee Anantakul, Parit Maimun, Pawita Tuithemwong, Phanusporn Moonnoo, Phetay Sakbunyuen, Pilaiwan Yodprasert, Pimwaree Jaiai, Pongpak Sananta, Pornpavee Saleesongsom, Pranee Srikampan, Prapatsorn Alongkornpradub, Ramida Burapa, Ramida Burapa, Rattanai Soparat, Ruekdee Kaewmuangma, Rutchawat Fakkaew, Sangdao Somsri, Saowalak Panyawong, Sarawin Phunyim, Sedtawut Kunnakulsoontorn, Sunirat Manemaung, Sunun Manowan, Supakit Anantakul, Supakorn Tulapong, Supanut Krittitasema, Supawadee Pongprapass, Suphakrit Jitrarat, Suporn Watanaporn, Sureepon Pratumma, Sutikarn Jarenjai, Suttipong Kawilapat, Tanakorn Khamwiang, Tanongsak Suriyakaew, Thanapol Wongkhamnan, Thanyalark Seeti, Theerapong Pinta, Thitipong Luecha, Tidarat Peungtonang, Torraporn Maneerat, Tunya Boonruean, Unyamanee Chantra, Visaka Ruankaew, Wachirapong Teerasawatt, Warakon Aranpitak, Warintorn Fakkaew, Warunee Srisuk, Wasana Chaiwong, Wasinee Poonpon, Watchara Kunthanang, Woottichai Sriwichai, Woranet Wangkhummuen, Woraphan Doungtip, Yatika Theptanee, Yokfah Meena, Yudtana Panyawong, Yupin Buain, Yuthana and Yuthaphom Hongsorn (outreach); Niphatta Mungkhala, Pongsak Pirom and Sakawrut Jitharidkul (hotline); Pra-ornsuda Sukrakanchana, Rukchanok Peongjakta and Suwalai Chalermpantmetagul (monitoring); Ampika Kaewbundit, Chutharat Kasemrat, Jiraporn Khamkon, Jutatip Kaewmalee, Laddawan Laomanit, Nantawan Wangsaeng, Patcharida Insee, Phornphimon Moolnoi, Pimpinun Punyathi, Rumpaiphorn Saisom and Thamonphat Phitak (laboratory); Aye Min Latt, Hark Murng, Naruporn Rawanchaikul and Sineenart Nupradit (translation); Kanchana Than-in-at, Marin Inta, Natthanidnan Sricharoen, Nirattiya Jaisieng, Ratchapat Jitharidkul, Sanuphong Chailoet and Wasan Wongwai (Data Center); Pongsak Pirom and Sakawrut Jitharidkul (administration); Ludovic Barra, Caroline Huron, Claire Le Moigne, Nitima Chaiboonruang, Thanchanok Sriwised, Thibaut Vallet, Tidarat Tritungtrakul (finance). Last but not least, we thank all study participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the ethics committees of the Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, and Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand. An Advisory Committee reviewed annually the study progress as well as the interim analysis results of the counseling study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salvadori, N., Decker, L., Ngo-Giang-Huong, N. et al. Impact of Counseling Methods on HIV Retesting Uptake in At-Risk Individuals: A Randomized Controlled Study. AIDS Behav 24, 1505–1516 (2020). https://doi.org/10.1007/s10461-019-02695-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-019-02695-2