Abstract
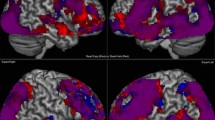
Clinical decision making requires knowledge, experience and analytical/non-analytical types of decision processes. As clinicians progress from novice to expert, research indicates decision-making becomes less reliant on foundational biomedical knowledge and more on previous experience. In this study, we investigated how knowledge and experience were reflected in terms of differences in neural areas of activation. Novice and expert clinicians diagnosed simple or complex (easy, hard) cases while functional magnetic resonance imaging (fMRI) data were collected. Our results highlight key differences in the neural areas activated in novices and experts during the clinical decision-making process. fMRI data were collected from ten second year medical students (novices) and ten practicing gastroenterologists (experts) while they diagnosed sixteen (eight easy and eight hard) clinical cases via multiple-choice questions. Behavioral data were collected for diagnostic accuracy (correct/incorrect diagnosis) and time taken to assign a clinical diagnosis. Two analyses were performed with the fMRI data. First, data from easy and hard cases were compared within respective groups (easy > hard, hard > easy). Second, neural differences between novices and experts (novice > expert, expert > novice) were assessed. Experts correctly diagnosed more cases than novices and made their diagnoses faster than novices on both easy and hard cases (all p’s < 0.05). Time taken to diagnose hard cases took significantly longer for both novices and experts. While similar neural areas were activated in both novices and experts during the decision making process, we identified significant hemispheric activation differences between novice and expert clinicians when diagnosing hard clinical cases. Specifically, novice clinicians had greater activations in the left anterior temporal cortex and left ventral lateral prefrontal cortex whereas expert clinicians had greater activations in the right dorsal lateral, right ventral lateral, and right parietal cortex. Hemispheric differences in activation were not observed between novices and experts while diagnosing easy clinical cases. While clinical decision-making engaged the prefrontal cortex (PFC) in both novices and experts, interestingly we observed expertise related differences in the regions and hemispheres of PFC activation between these groups for hard clinical cases. Specifically, in novices we observed activations in left hemisphere neural regions associated with factual rule-based knowledge, whereas in experts we observed right hemisphere activation in neural regions associated with experiential knowledge. Importantly, at the neural level, our data highlight differences in so called type 2 clinical decision-making processes related to prior knowledge and experience.


Similar content being viewed by others
Change history
25 July 2017
An erratum to this article has been published.
References
Amaro, E, Jr., & Barker, G. J. (2006). Study design in fMRI: Basic principles. Brain and Cognition, 60(3), 220–232.
Ashby, F. G. (2011). Statistical analysis of FMRI Data. Cambridge, MA: MIT Press.
Attwell, D., & Iadecola, C. (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25(12), 621–625.
Bahrami, P., Graham, S. J., Grantcharov, T. P., Cusimano, M. D., Rotstein, O. D., Mansur, A., & Schweizer, T. A. (2014). Neuroanatomical correlates of laparoscopic surgery training. Surgical Endoscopy, 28(7), 2189–2198.
Barbey, A. K., & Patterson, R. (2011). Architecture of explanatory inference in the human prefrontal cortex. Frontiers in Psychology, 2. doi:10.3389/fpsyg.2011.00162.
Bever, T. G., & Chiarello, R. J. (1974). Cerebral dominance in musicians and nonmusicians. Science, 185(4150), 537–539.
Cabeza, R., & Kingstone, A. (2006). Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press.
Coderre, S., Wright, B., & McLaughlin, K. (2010). To think is good: Querying an initial hypothesis reduces diagnostic error in medical students. Academic Medicine: Journal of the Association of American Medical Colleges, 85(7), 1125–1129.
Croskerry, P. (2005). The theory and practice of clinical decision-making. Canadian Journal of Anesthesia/Journal Canadian D’anesthésie, 52, R1–R8.
Croskerry, P. (2009a). A universal model of diagnostic reasoning. Academic Medicine: Journal of the Association of American Medical Colleges, 84(8), 1022–1028.
Croskerry, P. (2009b). Clinical cognition and diagnostic error: Applications of a dual process model of reasoning. Advances in Health Sciences Education, 14(S1), 27–35.
Durning, S. J., Graner, J., Artino, A. R., Pangaro, L. N., Beckman, T., Holmboe, E., et al. (2012). Using functional neuroimaging combined with a think-aloud protocol to explore clinical reasoning expertise in internal medicine. Military Medicine, 177(9 Suppl), 72–78.
Elstein, A., Shulman, L. S., & Sprafka, S. A. (1978). Medical problem solving: An analysis of clinical reasoning. Cambridge, MA: Harvard University Press.
Ericsson, K. A., Charness, N., Feltovich, P. J., & Hoffman, R. R. (2006). The Cambridge handbook of expertise and expert performance. New York, NY: Cambridge University Press.
Eva, K. W. (2005). What every teacher needs to know about clinical reasoning. Medical Education, 39(1), 98–106.
Evans, J. (2003). In two minds: Dual-process accounts of reasoning. Trends in Cognitive Sciences, 7(10), 454–459.
Evans, J. (2008). Dual-processing accounts of reasoning, judgment, and social cognition. Annual Review of Psychology, 59(1), 255–278.
Evans, J., & Stanovich, K. (2013). Dual-process theories of higher cognition: Advancing the debate. Perspectives on Psychological Science, 8(3), 223–241.
Fangmeier, T., Knauff, M., Ruff, C. C., & Sloutsky, V. (2006). FMRI evidence for a three-stage model of deductive reasoning. Journal of Cognitive Neuroscience, 18(3), 320–334.
Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain: A Journal of Neurology, 123(7), 1293–1326.
Goel, V. (2007). Anatomy of deductive reasoning. Trends in Cognitive Sciences, 11(10), 435–441.
Gruppen, L. D., & Frohna, A. Z. (2002). Clinical reasoning. In D. I. Newble (Ed.), International handbook of research in medical education (pp. 205–230). Dordrecht: Springer.
Heeger, D. J., & Ress, D. (2002). What does fMRI tell us about neuronal activity? Nature Reviews Neuroscience, 3(2), 142–151.
Hruska, P., Krigolson, O., Coderre, S., McLaughlin, K., Cortese, F., Doig, C., et al. (2015). Working memory, reasoning, and expertise in medicine - insights into their relationships using functional neuroimaging. Advances in Health Sciences Education. doi:10.1007/s1049-015-9649-2.
Huettel, S. A., Song, A. W., & McCarthy, G. (2009). Functional magnetic resonance imaging. Sunderland, MA: Sinauer Associates, Incorporated.
Krawczyk, D. C. (2002). Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews, 26(6), 631–664.
Larsen, D. P., Butler, A. C., & Roediger, H. L, I. I. I. (2013). Comparative effects of test-enhanced learning and self-explanation on long-term retention. Medical Education, 47(7), 674–682.
Lee, D., Rushworth, M. F. S., Walton, M. E., Watanabe, M., & Sakagami, M. (2007). Functional specialization of the primate frontal cortex during decision making. Journal of Neuroscience, 27(31), 8170–8173.
Logothetis, N. K. (2003). The underpinnings of the BOLD functional magnetic resonance imaging signal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(10), 3963–3971.
McElroy, T., & Seta, J. J. (2004). On the other hand am I rational? Hemispheric activation and the framing effect. Brain and Cognition, 55(3), 572–580.
Norman, G. (2005). Research in clinical reasoning: Past history and current trends. Medical Education, 39(4), 418–427.
Patel, V. L., Evans, D. A., & Groen, G. J. (1989). Biomedical knowledge and clinical reasoning. Cambridge, MA: MIT Press.
Pelaccia, T., Tardif, J., Triby, E., & Charlin, B. (2011). An analysis of clinical reasoning through a recent and comprehensive approach: The dual-process theory. Medical Education Online, 16. doi:10.3402/meo.v16i0.5890.
Poldrack, R. A. (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2(1), 67–70.
Raichle, M. E., & Mintun, M. A. (2006). Brain work and brain imaging. Annual Review of Neuroscience, 29(1), 449–476.
Raman, M., McLaughlin, K., Violato, C., Rostom, A., Allard, J. P., & Coderre, S. (2010). Teaching in small portions dispersed over time enhances long-term knowledge retention. Medical Teacher, 32(3), 250–255.
Rogers, T., Hocking, J., Noppeney, U., Mechelli, A., Gorno-Tempini, M., Patterson, K., & Price, C. (2006). Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive, Affective and Behavioral Neuroscience, 6(3), 201–213.
Savoy, R. L. (2001). History and future directions of human brain mapping and functional neuroimaging. Acta Psychologica, 107(1–3), 9–42.
Savoy, R. L. (2006). Using small numbers of subjects in fMRI-based research. IEEE: The Quarterly Magazine of the Engineering in Medicine and Biology Society, 25(2), 52–59.
Worsley, K. J., Evans, A. C., Marrett, S., & Neelin, P. (1992). A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism, 12, 900.
Worsley, K. J., Marrett, S., Neelin, P., Vandal, A. C., Friston, K. J., & Evans, A. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4(1), 58–73.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s10459-017-9787-9.
Appendix: fMRI basics
Appendix: fMRI basics
Functional magnetic resonance imaging (fMRI) images are made possible by tracking hemodynamic response to neural activity over time (Huettel et al. 2009; Logothetis 2003). When neurons become active in response to a task or demand, hemodynamic changes of increased blood volume, increased blood flow and alterations in oxygenation occur (Attwell and Iadecola 2002; Heeger and Ress 2002). These changes produce the blood oxygen level dependent (BOLD) signal, which can be simplistically described as a ratio of oxygenated to deoxygenated hemoglobin (Ashby 2011). The underlying assumption in fMRI is that increased oxyhemoglobin concentration indicates nearby neural activity (Savoy 2001).
A block design was chosen for this research, where tasks were presented in a sequential manner with alternating periods of stimulation and rest (Amaro and Barker 2006). Specifically, experimental blocks of clinical decision-making tasks (multiple choice questions), alternated with rest blocks called fixation periods. Fixation periods served as baseline, and can be thought of as a control condition during which no task is being performed (Raichle and Mintun 2006). In fixation periods, no text was presented and no response was expected from participants; it involved simply looking at a plus sign (fixation cross) on the screen for 10 s intervals.
To determine neural areas of activation in clinical decision making, averaged neural activity across fixation trials were contrasted to averaged neural activity in defined clinical decision making experimental blocks (answering MCQ). When differences in level of activation were found to be greater during the experimental phase of the task (i.e. during decision making) than activation during fixation (baseline/rest), neural activity is interpreted as being attributed to the cognitive process of clinical decision making (Amaro and Barker 2006).
Subject data obtained in this fMRI research are representative of each participant’s brain. These pieces of data are called voxels, analogous to 3D pixels, and are volumetric in nature (Ashby 2011). Each voxel is represented by 3D coordinates (x, y, z), which are used to identify associated structural areas using brain atlas tools.
Rights and permissions
About this article
Cite this article
Hruska, P., Hecker, K.G., Coderre, S. et al. Hemispheric activation differences in novice and expert clinicians during clinical decision making. Adv in Health Sci Educ 21, 921–933 (2016). https://doi.org/10.1007/s10459-015-9648-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10459-015-9648-3