Abstract
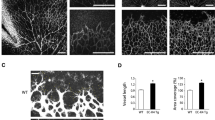
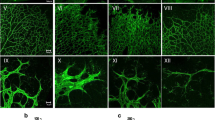
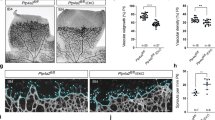
The Dll4-Notch-signaling pathway regulates capillary sprouting via the specification of endothelial tip cells. While VEGF is a potent inducer of Dll4 expression, the intracellular mediators that stimulate its expression remain poorly defined. The protein tyrosine phosphatase PTPRJ/DEP-1 is required for angiogenesis in normal or pathological contexts through its modulation of VEGF signaling. Here, we show that in DEP-1 KO mice, retinas at post-natal day 5 show enlarged blood vessels, as well as an increased number of tip cells and vessel branching points at the migrating front of the vascular plexus. Consistent with these observations, the proliferation of endothelial cells is increased in the retinas of DEP-1 KO mice, as revealed by phospho-histone H3 staining, and increased phosphorylation of ERK1/2 in HUVECs transfected with DEP-1 siRNA. The expression of Dll4 was decreased in retinas of DEP-1 KO mice and was associated with decreased Notch activation. Mechanistically, reduced Dll4 expression in the absence of DEP-1 was correlated with the inhibition of the Src/Akt/β-Catenin-signaling pathway in HUVECs. Conversely, overexpression of WT DEP-1 in cultured endothelial cells, but not of mutants unable to activate Src-dependent signaling, promoted Dll4 expression. Inhibition of Src, Akt, and β-catenin transcriptional activity, leading to the inhibition of Dll4 expression, further suggested that their activation through a DEP-1-dependent pathway was required to promote Dll4 expression in VEGF-stimulated endothelial cells. Altogether, these data demonstrate that DEP-1, via Akt and β-catenin, is a significant promoter of the VEGF-induced Dll4-Notch pathway, and can contribute to the regulation of the tip and stalk cell phenotypes of endothelial cells.





Similar content being viewed by others
References
Ostman A, Yang Q, Tonks NK (1994) Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc Natl Acad Sci USA 91:9680–9684
Lampugnani MG, Zanetti A, Corada M et al (2003) Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 161:793–804. https://doi.org/10.1083/jcb.200209019
Sacco F, Tinti M, Palma A et al (2009) Tumor suppressor density-enhanced phosphatase-1 (DEP-1) inhibits the RAS pathway by direct dephosphorylation of ERK1/2 kinases. J Biol Chem 284:22048–22058. https://doi.org/10.1074/jbc.M109.002758
Chabot C, Spring K, Gratton J-P et al (2009) New role for the protein tyrosine phosphatase DEP-1 in Akt activation and endothelial cell survival. Mol Cell Biol 29:241–253. https://doi.org/10.1128/MCB.01374-08
Takahashi T, Takahashi K, St John PL et al (2003) A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol Cell Biol 23:1817–1831
Rodriguez F, Vacaru A, Overvoorde J, den Hertog J (2008) The receptor protein-tyrosine phosphatase, Dep1, acts in arterial/venous cell fate decisions in zebrafish development. Dev Biol 324:122–130. https://doi.org/10.1016/j.ydbio.2008.09.011
Spring K, Chabot C, Langlois S et al (2012) Tyrosine phosphorylation of DEP-1/CD148 as a mechanism controlling Src kinase activation, endothelial cell permeability, invasion, and capillary formation. Blood 120:2745–2756. https://doi.org/10.1182/blood-2011-12-398040
Zhu JW, Doan K, Park J et al (2011) Receptor-like tyrosine phosphatases CD45 and CD148 have distinct functions in chemoattractant-mediated neutrophil migration and response to S. aureus. Immunity 35:757–769. https://doi.org/10.1016/j.immuni.2011.09.011
Trapasso F, Drusco A, Costinean S et al (2006) Genetic ablation of Ptprj, a mouse cancer susceptibility gene, results in normal growth and development and does not predispose to spontaneous tumorigenesis. DNA Cell Biol 25:376–382. https://doi.org/10.1089/dna.2006.25.376
Hackbusch D, Dulsner A, Gatzke N et al (2013) Knockout of density-enhanced phosphatase-1 impairs cerebrovascular reserve capacity in an arteriogenesis model in mice. BioMed Res Int 2013:802149. https://doi.org/10.1155/2013/802149
Zhu JW, Brdicka T, Katsumoto TR et al (2008) Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity 28:183–196. https://doi.org/10.1016/j.immuni.2007.11.024
Stepanek O, Kalina T, Draber P et al (2011) Regulation of Src family kinases involved in T cell receptor signaling by protein-tyrosine phosphatase CD148. J Biol Chem 286:22101–22112. https://doi.org/10.1074/jbc.M110.196733
Ellison S, Mori J, Barr AJ, Senis YA (2010) CD148 enhances platelet responsiveness to collagen by maintaining a pool of active Src family kinases. J Thromb Haemost JTH 8:1575–1583. https://doi.org/10.1111/j.1538-7836.2010.03865.x
Fournier P, Dussault S, Fusco A et al (2016) Tyrosine phosphatase PTPRJ/DEP-1 is an essential promoter of vascular permeability, angiogenesis, and tumor progression. Cancer Res 76:5080–5091. https://doi.org/10.1158/0008-5472.CAN-16-1071
Geudens I, Gerhardt H (2011) Coordinating cell behaviour during blood vessel formation. Dev Camb Engl 138:4569–4583. https://doi.org/10.1242/dev.062323
Gerhardt H, Golding M, Fruttiger M et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177. https://doi.org/10.1083/jcb.200302047
Blancas AA, Wong LE, Glaser DE, McCloskey KE (2013) Specialized tip/stalk-like and phalanx-like endothelial cells from embryonic stem cells. Stem Cells Dev 22:1398–1407. https://doi.org/10.1089/scd.2012.0376
Jakobsson L, Franco CA, Bentley K et al (2010) Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12:943–953. https://doi.org/10.1038/ncb2103
Lobov IB, Renard RA, Papadopoulos N et al (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104:3219–3224. https://doi.org/10.1073/pnas.0611206104
Suchting S, Freitas C, le Noble F et al (2007) The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104:3225–3230. https://doi.org/10.1073/pnas.0611177104
Hellstrom M, Phng L-K, Hofmann JJ et al (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445:776–780. https://doi.org/10.1038/nature05571
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci CMLS 66:1631–1646. https://doi.org/10.1007/s00018-009-8668-7
Leslie JD, Ariza-McNaughton L, Bermange AL et al (2007) Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Dev Camb Engl 134:839–844. https://doi.org/10.1242/dev.003244
Williams CK, Li J-L, Murga M et al (2006) Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 107:931–939. https://doi.org/10.1182/blood-2005-03-1000
Wu Y, Cain-Hom C, Choy L et al (2010) Therapeutic antibody targeting of individual Notch receptors. Nature 464:1052–1057. https://doi.org/10.1038/nature08878
Funahashi Y, Hernandez SL, Das I et al (2008) A Notch1 ectodomain construct inhibits endothelial Notch signaling, tumor growth, and angiogenesis. Cancer Res 68:4727–4735. https://doi.org/10.1158/0008-5472.CAN-07-6499
Thurston G, Noguera-Troise I, Yancopoulos GD (2007) The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 7:327–331. https://doi.org/10.1038/nrc2130
Limbourg FP, Takeshita K, Radtke F et al (2005) Essential role of endothelial Notch1 in angiogenesis. Circulation 111:1826–1832. https://doi.org/10.1161/01.CIR.0000160870.93058.DD
Krebs LT, Shutter JR, Tanigaki K et al (2004) Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18:2469–2473. https://doi.org/10.1101/gad.1239204
Gale NW, Dominguez MG, Noguera I et al (2004) Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101:15949–15954. https://doi.org/10.1073/pnas.0407290101
Ridgway J, Zhang G, Wu Y et al (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–1087. https://doi.org/10.1038/nature05313
Noguera-Troise I, Daly C, Papadopoulos NJ et al (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444:1032–1037. https://doi.org/10.1038/nature05355
Corada M, Nyqvist D, Orsenigo F et al (2010) The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell 18:938–949. https://doi.org/10.1016/j.devcel.2010.05.006
Larrivee B, Prahst C, Gordon E et al (2012) ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22:489–500. https://doi.org/10.1016/j.devcel.2012.02.005
Spring K, Lapointe L, Caron C et al (2014) Phosphorylation of DEP-1/PTPRJ on threonine 1318 regulates Src activation and endothelial cell permeability induced by vascular endothelial growth factor. Cell Signal 26:1283–1293. https://doi.org/10.1016/j.cellsig.2014.02.008
Nowak-Sliwinska P, Alitalo K, Allen E et al (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21:425–532. https://doi.org/10.1007/s10456-018-9613-x
Takahashi T, Yamaguchi S, Chida K, Shibuya M (2001) A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J 20:2768–2778. https://doi.org/10.1093/emboj/20.11.2768
Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17:611–625. https://doi.org/10.1038/nrm.2016.87
Villa N, Walker L, Lindsell CE et al (2001) Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108:161–164
Takeshita K, Satoh M, Ii M et al (2007) Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res 100:70–78. https://doi.org/10.1161/01.RES.0000254788.47304.6e
Liu Z-J, Shirakawa T, Li Y et al (2003) Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23:14–25
Fang D, Hawke D, Zheng Y et al (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282:11221–11229. https://doi.org/10.1074/jbc.M611871200
Monick MM, Carter AB, Robeff PK et al (2001) Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J Immunol 1950 166:4713–4720
Stone J, Itin A, Alon T et al (1995) Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci Off J Soc Neurosci 15:4738–4747
Maes C, Goossens S, Bartunkova S et al (2010) Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J 29:424–441. https://doi.org/10.1038/emboj.2009.361
Provis JM, Leech J, Diaz CM et al (1997) Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res 65:555–568. https://doi.org/10.1006/exer.1997.0365
Sebio A, Kahn M, Lenz H-J (2014) The potential of targeting Wnt/beta-catenin in colon cancer. Expert Opin Ther Targets 18:611–615. https://doi.org/10.1517/14728222.2014.906580
Cross DA, Alessi DR, Cohen P et al (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. https://doi.org/10.1038/378785a0
Trindade A, Kumar SR, Scehnet JS et al (2008) Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood 112:1720–1729. https://doi.org/10.1182/blood-2007-09-112748
Paduano F, Ortuso F, Campiglia P et al (2012) Isolation and functional characterization of peptide agonists of PTPRJ, a tyrosine phosphatase receptor endowed with tumor suppressor activity. ACS Chem Biol 7:1666–1676. https://doi.org/10.1021/cb300281t
Brunner PM, Heier PC, Mihaly-Bison J et al (2011) Density enhanced phosphatase-1 down-regulates urokinase receptor surface expression in confluent endothelial cells. Blood 117:4154–4161. https://doi.org/10.1182/blood-2010-09-307694
Takahashi T, Takahashi K, Mernaugh RL et al (2006) A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood 108:1234–1242. https://doi.org/10.1182/blood-2005-10-4296
Ren B, Deng Y, Mukhopadhyay A et al (2010) ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest 120:1217–1228. https://doi.org/10.1172/JCI39837
Sainson RCA, Aoto J, Nakatsu MN et al (2005) Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J Off Publ Fed Am Soc Exp Biol 19:1027–1029. https://doi.org/10.1096/fj.04-3172fje
Liu Z-J, Xiao M, Balint K et al (2006) Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J Off Publ Fed Am Soc Exp Biol 20:1009–1011. https://doi.org/10.1096/fj.05-4880fje
Srinivasan R, Zabuawala T, Huang H et al (2009) Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 4:e8283. https://doi.org/10.1371/journal.pone.0008283
Bullard LE, Qi X, Penn JS (2003) Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci 44:1722–1731
Yang C, Guo Y, Jadlowiec CC et al (2013) Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells. J Surg Res 183:478–486. https://doi.org/10.1016/j.jss.2013.01.009
Watson O, Novodvorsky P, Gray C et al (2013) Blood flow suppresses vascular Notch signalling via dll4 and is required for angiogenesis in response to hypoxic signalling. Cardiovasc Res 100:252–261. https://doi.org/10.1093/cvr/cvt170
Sacilotto N, Monteiro R, Fritzsche M et al (2013) Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA 110:11893–11898. https://doi.org/10.1073/pnas.1300805110
Acknowledgements
We would like to thank A. Fusco for providing DEP-1 KO mice. This work was supported by operating grants from the Cancer Research Society (I.R. and B.L.), an operating grant from the Canadian Institutes of Health Research (363450) (B.L.) and a Grant-in-Aid from the Heart and Stroke Foundation of Canada (B.L.). B.L. is the recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada. P. Fournier was supported by student scholarships from the Canadian Institutes of Health Research (292353) and Institut du cancer de Montréal.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fournier, P., Viallard, C., Dejda, A. et al. The protein tyrosine phosphatase PTPRJ/DEP-1 contributes to the regulation of the Notch-signaling pathway and sprouting angiogenesis. Angiogenesis 23, 145–157 (2020). https://doi.org/10.1007/s10456-019-09683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09683-z