Abstract
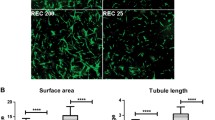
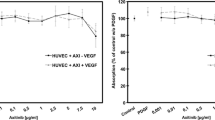
Proliferative diabetic retinopathy (PDR) represents a main cause of acquired blindness. Despite the recognition of the key role exerted by vascular endothelial growth factor (VEGF) in the pathogenesis of PDR, limitations to anti-VEGF therapies do exist. Thus, rapid and cost-effective angiogenesis assays are crucial for the screening of anti-angiogenic drug candidates for PDR therapy. In this context, evaluation of the angiogenic potential of PDR vitreous fluid may represent a valuable tool for preclinical assessment of angiostatic molecules. Here, vitreous fluid obtained from PDR patients after pars plana vitrectomy was used as a pro-angiogenic stimulus in a 3D endothelial cell spheroid/human vitreous assay. The results show that PDR vitreous is able to stimulate the sprouting of fibrin-embedded HUVEC spheroids in a time- and dose-dependent manner. A remarkable variability was observed among 40 individual vitreous fluid samples in terms of sprouting-inducing activity that was related, at least in part, to defined clinical features of the PDR patient. This activity was hampered by various extracellular and intracellular signaling pathway inhibitors, including the VEGF antagonist ranibizumab. When tested on 20 individual vitreous fluid samples, the inhibitory activity of ranibizumab ranged between 0 and 100% of the activity measured in the absence of the drug, reflecting a variable contribution of angiogenic mediators distinct from VEGF. In conclusion, the 3D endothelial cell spheroid/human vitreous assay represents a rapid and cost-effective experimental procedure suitable for the evaluation of the anti-angiogenic activity of novel extracellular and intracellular drug candidates, with possible implications for the therapy of PDR.




Similar content being viewed by others
References
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Gariano RF, Gardner TW (2005) Retinal angiogenesis in development and disease. Nature 438:960–966. doi:10.1038/nature04482
Siemerink MJ, Augustin AJ, Schlingemann RO (2010) Mechanisms of ocular angiogenesis and its molecular mediators. Dev Ophthalmol 46:4–20. doi:10.1159/000320006
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366:1227–1239. doi:10.1056/NEJMra1005073
Kim LA, D’Amore PA (2012) A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol 181:376–379. doi:10.1016/j.ajpath.2012.06.006
Miller JW, Le Couter J, Strauss EC, Ferrara N (2013) Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120:106–114. doi:10.1016/j.ophtha.2012.07.038
Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL et al (1998) 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem 273:20556–20567
Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA et al (2005) Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112:1048–1053. doi:10.1016/j.ophtha.2005.01.043
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L et al (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99:11393–11398
Simunovic MP, Maberley DA (2015) Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina 35:1931–1942. doi:10.1097/IAE.0000000000000723
Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS (2012) Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol 130:1145–1152. doi:10.1001/archophthalmol.2012.1043
Ip MS, Domalpally A, Sun JK, Ehrlich JS (2015) Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122:367–374. doi:10.1016/j.ophtha.2014.08.048
Kieran MW, Kalluri R, Cho YJ (2012) The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2:a006593. doi:10.1101/cshperspect.a006593
van Wijngaarden P, Qureshi SH (2008) Inhibitors of vascular endothelial growth factor (VEGF) in the management of neovascular age-related macular degeneration: a review of current practice. Clin Exp Optom 91:427–437. doi:10.1111/j.1444-0938.2008.00305.x
Wang S, Park JK, Duh EJ (2012) Novel targets against retinal angiogenesis in diabetic retinopathy. Curr Diab Rep 12:355–363. doi:10.1007/s11892-012-0289-0
Sherris D (2007) Ocular drug development—future directions. Angiogenesis 10:71–76. doi:10.1007/s10456-007-9068-y
Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C (2006) Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2:71–98
Semeraro F, Cancarini A, dell’Omo R, Rezzola S, Romano MR et al (2015) Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res 2015:582060. doi:10.1155/2015/582060
Dal Monte M, Rezzola S, Cammalleri M, Belleri M, Locri F et al (2015) Antiangiogenic effectiveness of the urokinase receptor-derived peptide UPARANT in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 56:2392–2407. doi:10.1167/iovs.14-16323
Rezzola S, Corsini M, Chiodelli P, Cancarini A, Nawaz IM et al (2017) Inflammation and N-formyl peptide receptors mediate the angiogenic activity of human vitreous humour in proliferative diabetic retinopathy. Diabetologia. doi:10.1007/s00125-016-4204-0
Rezzola S, Dal Monte M, Belleri M, Bugatti A, Chiodelli P et al (2015) Therapeutic potential of anti-angiogenic multitarget N, O-sulfated E. coli K5 polysaccharide in diabetic retinopathy. Diabetes 64:2581–2592. doi:10.2337/db14-1378
Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J et al (2005) Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 353:782–792. doi:10.1056/NEJMoa041773
Yang J, Klassen H, Pries M, Wang W, Nissen MH (2009) Vitreous humor and albumin augment the proliferation of cultured retinal precursor cells. J Neurosci Res 87:495–502. doi:10.1002/jnr.21873
Murugeswari P, Shukla D, Kim R, Namperumalsamy P, Stitt AW et al (2014) Angiogenic potential of vitreous from Proliferative Diabetic Retinopathy and Eales’ Disease patients. PLoS ONE 9:e107551. doi:10.1371/journal.pone.0107551
Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N (2003) Angiogenesis assays: a critical overview. Clin Chem 49:32–40
Morin KT, Tranquillo RT (2013) In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp Cell Res 319:2409–2417. doi:10.1016/j.yexcr.2013.06.006
Rezzola S, Belleri M, Gariano G, Ribatti D, Costagliola C et al (2014) In vitro and ex vivo retina angiogenesis assays. Angiogenesis 17:429–442. doi:10.1007/s10456-013-9398-x
Rezzola S, Belleri M, Ribatti D, Costagliola C, Presta M et al (2013) A novel ex vivo murine retina angiogenesis (EMRA) assay. Exp Eye Res 112:51–56. doi:10.1016/j.exer.2013.04.014
Rezzola S, Paganini G, Semeraro F, Presta M, Tobia C (2016) Zebrafish (Danio rerio) embryo as a platform for the identification of novel angiogenesis inhibitors of retinal vascular diseases. Biochim Biophys Acta 1862:1291–1296. doi:10.1016/j.bbadis.2016.04.009
Heiss M, Hellstrom M, Kalen M, May T, Weber H et al (2015) Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J 29:3076–3084. doi:10.1096/fj.14-267633
Stroup WW (2012) Generalized linear mixed models: modern concepts, methods and application. CRC Press, Boca Raton
Team RC (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Thurn KT, Thomas S, Moore A, Munster PN (2011) Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol 7:263–283. doi:10.2217/fon.11.2
Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B et al (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122:477–485. doi:10.1001/archopht.122.4.477
Klein BE (2007) Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 14:179–183. doi:10.1080/09286580701396720
Tranos P, Vakalis A, Asteriadis S, Koukoula S, Vachtsevanos A et al (2013) Resistance to antivascular endothelial growth factor treatment in age-related macular de generation. Drug Des Dev Ther 7:485–490
Dedania VS, Bakri SJ (2016) Systemic safety of intravitreal anti-vascular endothelial growth factor agents in age-related macular degeneration. Curr Opin Ophthalmol 27:224–243. doi:10.1097/ICU.0000000000000257
Yang S, Zhao J, Sun X (2016) Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 10:1857–1867. doi:10.2147/DDDT.S97653
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. doi:10.1056/NEJM199412013312203
Takagi H, Watanabe D, Suzuma K, Kurimoto M, Suzuma I et al (2007) Novel role of erythropoietin in proliferative diabetic retinopathy. Diabetes Res Clin Pract 77(Suppl 1):S62–S64. doi:10.1016/j.diabres.2007.01.035
Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17:611–625. doi:10.1038/nrm.2016.87
Prakash CR, Raja S (2012) Indolinones as promising scaffold as kinase inhibitors: a review. Mini Rev Med Chem 12:98–119
Ghosh S, Chatterjee S (2013) Molecular blockade of angiogenic factors: a new therapeutic tool for the treatment of abnormal uterine bleeding. J Midlife Health 4:66–67. doi:10.4103/0976-7800.109647
Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY (2016) Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol 23:209–222. doi:10.1080/09286586.2016.1193618
Zhao C, Wang W, Xu D, Li H, Li M et al (2014) Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn Pathol 9:130. doi:10.1186/1746-1596-9-130
Benarous R, Sasongko MB, Qureshi S, Fenwick E, Dirani M et al (2011) Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci 52:7464–7469. doi:10.1167/iovs.11-7598
Nouhravesh N, Andersen HU, Jensen JS, Rossing P, Jensen MT (2016) Retinopathy is associated with impaired myocardial function assessed by advanced echocardiography in type 1 diabetes patients—The Thousand & 1 Study. Diabetes Res Clin Pract 116:263–269. doi:10.1016/j.diabres.2016.04.024
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S et al (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119:789–801. doi:10.1016/j.ophtha.2011.12.039
Shih T, Lindley C (2006) Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 28:1779–1802. doi:10.1016/j.clinthera.2006.11.015
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. doi:10.1097/01.iae.0000242842.14624.e7
Ronca R, Giacomini A, Di Salle E, Coltrini D, Pagano K et al (2015) Long-pentraxin 3 derivative as a small-molecule FGF trap for cancer therapy. Cancer Cell 28:225–239. doi:10.1016/j.ccell.2015.07.002
Gagliardi A, Hadd H, Collins DC (1992) Inhibition of angiogenesis by suramin. Cancer Res 52:5073–5075
Fong TA, Shawver LK, Sun L, Tang C, App H et al (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J et al (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5:835–844. doi:10.1038/nrd2130
Dimitroff CJ, Klohs W, Sharma A, Pera P, Driscoll D et al (1999) Anti-angiogenic activity of selected receptor tyrosine kinase inhibitors, PD166285 and PD173074: implications for combination treatment with photodynamic therapy. Invest New Drugs 17:121–135
Mohammadi M, McMahon G, Sun L, Tang C, Hirth P et al (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276:955–960
Al-Obeidi FA, Lam KS (2000) Development of inhibitors for protein tyrosine kinases. Oncogene 19:5690–5701. doi:10.1038/sj.onc.1203926
Alvarez Y, Astudillo O, Jensen L, Reynolds AL, Waghorne N et al (2009) Selective inhibition of retinal angiogenesis by targeting PI3 kinase. PLoS ONE 4:e7867. doi:10.1371/journal.pone.0007867
Giuliani N, Lunghi P, Morandi F, Colla S, Bonomini S et al (2004) Downmodulation of ERK protein kinase activity inhibits VEGF secretion by human myeloma cells and myeloma-induced angiogenesis. Leukemia 18:628–635. doi:10.1038/sj.leu.2403269
Schenone S, Manetti F, Botta M (2007) SRC inhibitors and angiogenesis. Curr Pharm Des 13:2118–2128
Zhou G, Myers R, Li Y, Chen Y, Shen X et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174. doi:10.1172/JCI13505
Yagasaki R, Nakahara T, Ushikubo H, Mori A, Sakamoto K et al (2014) Anti-angiogenic effects of mammalian target of rapamycin inhibitors in a mouse model of oxygen-induced retinopathy. Biol Pharm Bull 37:1838–1842
Mabeta P, Pepper MS (2009) A comparative study on the anti-angiogenic effects of DNA-damaging and cytoskeletal-disrupting agents. Angiogenesis 12:81–90. doi:10.1007/s10456-009-9134-8
Griggs J, Brindle KM, Metcalfe JC, Hill SA, Smith GA et al (2001) Potent anti-metastatic activity of combretastatin-A4. Int J Oncol 19:821–825
Eliceiri BP, Klemke R, Stromblad S, Cheresh DA (1998) Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol 140:1255–1263
Gahr S, Mayr C, Kiesslich T, Illig R, Neureiter D et al (2015) The pan-deacetylase inhibitor panobinostat affects angiogenesis in hepatocellular carcinoma models via modulation of CTGF expression. Int J Oncol 47:963–970. doi:10.3892/ijo.2015.3087
Acknowledgements
This work was supported in part by grants from Associazione Italiana per la Ricerca sul Cancro (IG AIRC Grant no. 18493) to M.P., from University of Brescia (H&W Starting Project 2015) to F.S. and from Novartis Farma S.p.A to F.S. and M.P.; S.R. was supported by a fellowship from AIRC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rezzola, S., Nawaz, I.M., Cancarini, A. et al. 3D endothelial cell spheroid/human vitreous humor assay for the characterization of anti-angiogenic inhibitors for the treatment of proliferative diabetic retinopathy. Angiogenesis 20, 629–640 (2017). https://doi.org/10.1007/s10456-017-9575-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9575-4