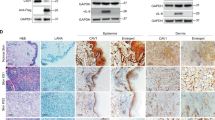
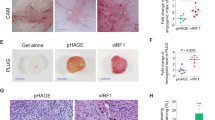
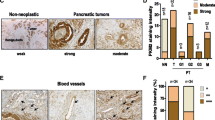
Abstract
Kaposi’s sarcoma (KS) is a vascular neoplasm caused by infection of endothelial or endothelial precursor cells with the Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV8). Research efforts have focused on defining the molecular events explaining how KSHV promotes pathological angiogenesis and KS tumor formation. mTOR/HIF-1 is a fundamental pathway driving these processes through the upregulation of angiogenic and inflammatory proteins, including VEGF, ANGPTL4, and ANGPT2. Interestingly, HIF-1 has also been implicated in the upregulation of metabolic genes associated with aerobic glycolysis and the growth of solid tumors. However, whether HIF-1 plays a role in regulating cell metabolism in KS remains unexplored. Here, we show that the HIF-1 metabolic effector, pyruvate kinase 2 (PKM2), is upregulated upon KSHV infection of endothelial cells and is necessary to maintain aerobic glycolysis in infected cells. We further demonstrate that PKM2 regulates KS angiogenic phenotype by acting as a coactivator of HIF-1 and increasing the levels of HIF-1 angiogenic factors, including VEGF. Indeed, inhibition of PKM2 expression blocked endothelial cell migration and differentiation and the angiogenic potential of KSHV-infected cells. We also investigated whether PKM2 regulates the angiogenic dysregulation induced by the KSHV-encoded G protein-coupled receptor (vGPCR), a viral oncogene that promotes Kaposi’s sarcomagenesis through the upregulation of HIF angiogenic factors. Interestingly, we found that PKM2 controls vGPCR-induced VEGF paracrine secretion and vGPCR oncogenesis. Our findings provide a molecular mechanism for how HIF-1 dysregulation fuels both angiogenesis and tumor metabolism in KS and support further investigations on therapeutic approaches targeting HIF-1 and PKM2 for KS treatment.






Similar content being viewed by others
References
Moore PS, Chang Y (2001) Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci 356(1408):499–516
Mesri EA, Cesarman E, Boshoff C (2010) Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer 10(10):707–719
Jham BC, Montaner S (2010) The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor: lessons on dysregulated angiogenesis from a viral oncogene. J Cell Biochem 110(1):1–9
Jham BCMT, Hu J, Chaisuparat R, Friedman ER, Pandolfi PP, Schneider A, Sodhi A, Montaner S (2011) Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi’s sarcoma. PLoS One 6(4):e19103
Carroll PA, Kenerson HL, Yeung RS, Lagunoff M (2006) Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol 80(21):10802–10812
Catrina SB, Botusan IR, Rantanen A, Catrina AI, Pyakurel P, Savu O, Axelson M, Biberfeld P, Poellinger L, Brismar K (2006) Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2 alpha are expressed in kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res 12(15):4506–4514
Shin YC, Joo CH, Gack MU, Lee HR, Jung JU (2008) Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res 68(6):1751–1759
Cai QL, Knight JS, Verma SC, Zald P, Robertson ES (2006) EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog 2(10):e116
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29(5):625–634
Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S (2006) The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 10(2):133–143
Ma T, Jham BC, Hu J, Friedman ER, Basile JR, Molinolo A, Sodhi A, Montaner S (2010) Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. Proc Natl Acad Sci U S A 107(32):14363–14368
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20(1):51–56
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW (2011) Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol 76:325–334
Mazurek S, Boschek CB, Hugo F, Eigenbrodt E (2005) Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15(4):300–308
Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC (2010) Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329(5998):1492–1499
Chaneton B, Gottlieb E (2012) Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci 37(8):309–316
Luo W, Semenza GL (2012) Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab 23(11):560–566
Tamada M, Suematsu M, Saya H (2012) Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 18(20):5554–5561
Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145(5):732–744
Vieira J, O’Hearn PM (2004) Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology 325(2):225–240
Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, Lagunoff M (2010) Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc Natl Acad Sci U S A 107(23):10696–10701
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11(5):407–420
Li Z, Yang P (1846) Li Z (2014) The multifaceted regulation and functions of PKM2 in tumor progression. Biochim Biophys Acta 2:285–296
Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG (2003) Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol 162(5):1431–1439
Cai Q, Murakami M, Si H, Robertson ES (2007) A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposi’s sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia. J Virol 81(19):10413–10423
Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS (2000) The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res 60(17):4873–4880
Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS (2003) Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3(1):23–36
Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET, Gutkind JS (2006) The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi’s sarcoma. Cancer Res 66(1):168–174
Semenza GL (2012) Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33(4):207–214
Laplante M, Sabatini DM (2013) Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126(Pt 8):1713–1719
Veeranna RP, Haque M, Davis DA, Yang M, Yarchoan R (2012) Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induction by hypoxia and hypoxia-inducible factors. J Virol 86(2):1097–1108
Yogev O, Lagos D, Enver T, Boshoff C (2014) Kaposi’s sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathog 10(9):e1004400
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452(7184):230–233
Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8(10):839–847
Dittmer DP, Richards KL, Damania B (2012) Treatment of Kaposi sarcoma-associated herpesvirus-associated cancers. Front Microbiol 3:141
Yarchoan R, Pluda JM, Wyvill KM, Aleman K, Rodriguez-Chavez IR, Tosato G, Catanzaro AT, Steinberg SM, Little RF (2007) Treatment of AIDS-related Kaposi’s sarcoma with interleukin-12: rationale and preliminary evidence of clinical activity. Crit Rev Immunol 27(5):401–414
Uldrick TS, Polizzotto MN, Yarchoan R (2012) Recent advances in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Curr Opin Oncol 24(5):495–505
Krown SE, Roy D, Lee JY, Dezube BJ, Reid EG, Venkataramanan R, Han K, Cesarman E, Dittmer DP (2012) Rapamycin with antiretroviral therapy in AIDS-associated Kaposi sarcoma: an AIDS Malignancy Consortium study. J Acquir Immune Defic Syndr 59(5):447–454
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Venegas V, Wang J, Dimmock D, Wong LJ (2011) Real-time quantitative PCR analysis of mitochondrial DNA content. In: Haines JL et al. Current protocols in human genetics/editorial board, Chap 19:Unit 19 17
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, T., Patel, H., Babapoor-Farrokhran, S. et al. KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi’s sarcoma. Angiogenesis 18, 477–488 (2015). https://doi.org/10.1007/s10456-015-9475-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-015-9475-4