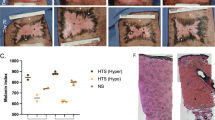
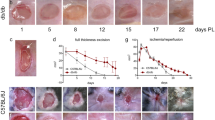
Abstract
Impaired wound healing is a persistent clinical problem which has been treated with mixed results. Studies aimed at elucidating the mechanism of impaired wound healing have focused on small cohorts of genes which leave an incomplete picture of the wound healing process. We aimed to investigate impaired wound healing via a comprehensive panel of angiogenic/inflammation-related genes and wound closure kinetics with and without the application of extracorporeal shock wave therapy (ESWT), which has been demonstrated to improve wound healing. Full-thickness skin from the dorsal surface of “normal” (BALB/c) and “impaired” (db +/db +) mice was excised, and wound margin tissue was harvested 2, 7, and 10 days post injury. A separate, but identical wound model was established over 40 days in order to measure wound closure kinetics. Over time, the normal non-ESWT treated wounds exhibited varying patterns of elevated expression of 25–30 genes, whereas wounds with impaired healing displayed prolonged elevated expression of only a few genes (CXCL2, CXCL5, CSF3, MMP9, TGF-α). In response to ESWT, gene expression was augmented in both types of wounds, especially in the expression of PECAM-1; however, ESWT had no effect on wound closure in either model. In addition, multiple doses of ESWT exacerbated the delayed wound healing, and actually caused the wounds to initially increase in size. These data provide a more complete picture of impaired wound healing, and a way to evaluate various promising treatments.




Similar content being viewed by others
References
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276(5309):75–81
Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF (2007) Impaired wound healing. Clin Dermatol 25(1):19–25
Bauer SM, Bauer RJ, Velazquez OC (2005) Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg 39(4):293–306
Ochoa O, Torres FM, Shireman PK (2007) Chemokines and diabetic wound healing. Vascular 15(6):350–355
Mast BA, Schultz GS (1996) Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 4(4):411–420
Broughton G II, Janis JE, Attinger CE (2006) Wound healing: an overview. Plast Reconstr Surg 117(7 Suppl):1e-S–32e-S
Mullner T, Mrkonjic L, Kwasny O, Vecsei V (1997) The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 50(3):194–199
Franz MG, Steed DL, Robson MC (2007) Optimizing healing of the acute wound by minimizing complications. Curr Probl Surg 44(11):691–763
Neal MS (2001) Angiogenesis: is it the key to controlling the healing process? J Wound Care 10(7):281–287
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S (2004) CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol 25(4):201–209
Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O (2003) Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med 47(3):149–161
Callaghan MJ, Chang EI, Seiser N et al (2008) Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release. Plast Reconstr Surg 121(1):130–141
Cianfarani F, Zambruno G, Brogelli L et al (2006) Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am J Pathol 169(4):1167–1182
Wang CJ (2003) An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J 26(4):220–232
Yan X, Zeng B, Chai Y, Luo C, Li X (2008) Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg 61(6):646–653
Nishida T, Shimokawa H, Oi K et al (2004) Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 110(19):3055–3061
Stojadinovic A, Elster EA, Anam K et al (2008) Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 11(4):369–380
Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S (2006) Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 114(25):2823–2830
Meirer R, Brunner A, Deibl M, Oehlbauer M, Piza-Katzer H, Kamelger FS (2007) Shock wave therapy reduces necrotic flap zones and induces VEGF expression in animal epigastric skin flap model. J Reconstr Microsurg 23(4):231–236
Davis TA, Stojadinovic A, Anam K et al (2009) Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int Wound J 6(1):11–21
Kuo YR, Wang CT, Wang FS, Yang KD, Chiang YC, Wang CJ (2009) Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen 17(1):80–87
Kuo YR, Wu WS, Hsieh YL et al (2007) Extracorporeal shock wave enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J Surg Res 143(2):385–392
Schaden W, Thiele R, Kolpl C et al (2007) Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res 143(1):1–12
Ennis WJ, Lee C, Meneses P (2007) A biochemical approach to wound healing through the use of modalities. Clin Dermatol 25(1):63–72
Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. Faseb J 20(7):811–827
Kavros SJ, Liedl DA, Boon AJ, Miller JL, Hobbs JA, Andrews KL (2008) Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv Skin Wound Care 21(9):416–423
Walker NA, Denegar CR, Preische J (2007) Low-intensity pulsed ultrasound and pulsed electromagnetic field in the treatment of tibial fractures: a systematic review. J Athl Train 42(4):530–535
Kavros SJ, Miller JL, Hanna SW (2007) Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo clinic experience, 2004–2006. Adv Skin Wound Care 20(4):221–226
Fukumoto Y, Ito A, Uwatoku T et al (2006) Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 17(1):63–70
Rompe JD, Zoellner J, Nafe B (2001) Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res 387:72–82
Kikuchi Y, Ito K, Ito Y et al (2010) Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J 74(3):589–591
Schaden W, Fischer A, Sailler A (2001) Extracorporeal shock wave therapy of nonunion or delayed osseous union. Clin Orthop Relat Res 387:90–94
Belperio JA, Keane MP, Arenberg DA et al (2000) CXC chemokines in angiogenesis. J Leukoc Biol 68(1):1–8
Rosenthal A, Lindquist PB, Bringman TS, Goeddel DV, Derynck R (1986) Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell 46(2):301–309
Schreiber AB, Winkler ME, Derynck R (1986) Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science 232(4755):1250–1253
Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE (1993) Expression of growth factor and receptor mRNAs in skin epithelial cells following acute cutaneous injury. Am J Pathol 142(4):1099–1110
Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC (1993) TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73(2):263–278
Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR (1993) Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73(2):249–261
DeLisser HM, Christofidou-Solomidou M, Strieter RM et al (1997) Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 151(3):671–677
Fujiwara K (2006) Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259(4):373–380
Li S, Huang NF, Hsu S (2005) Mechanotransduction in endothelial cell migration. J Cell Biochem 96(6):1110–1126
Greene AK, Puder M, Roy R et al (2006) Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 56(4):418–422
Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP (2004) Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 114(5):1086–1096 (discussion 1097–1088)
Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4(8):617–629
McCawley LJ, O’Brien P, Hudson LG (1998) Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J Cell Physiol 176(2):255–265
Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A (2005) Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors. Mol Cell Biochem 269(1–2):209–216
Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G (2003) Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem 270(18):3739–3749
Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G (2002) Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol 119(1):91–98
Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK (1996) Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 107(5):743–748
Wysocki AB, Staiano-Coico L, Grinnell F (1993) Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 101(1):64–68
Cao R, Brakenhielm E, Pawliuk R et al (2003) Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med 9(5):604–613
Miller DL, Ortega S, Bashayan O, Basch R, Basilico C (2000) Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol 20(6):2260–2268
Aggarwal VS, Liao J, Bondarev A et al (2006) Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet 15(21):3219–3228
Mitsiadis TA, Tucker AS, De Bari C, Cobourne MT, Rice DP (2008) A regulatory relationship between Tbx1 and FGF signaling during tooth morphogenesis and ameloblast lineage determination. Dev Biol 320(1):39–48
Lania G, Zhang Z, Huynh T et al (2009) Early thyroid development requires a Tbx1-Fgf8 pathway. Dev Biol 328(1):109–117
Terasaki K, Kanzaki T, Aoki T, Iwata K, Saiki I (2003) Effects of recombinant human tissue inhibitor of metalloproteinases-2 (rh-TIMP-2) on migration of epidermal keratinocytes in vitro and wound healing in vivo. J Dermatol 30(3):165–172
Kzhyshkowska J, Workman G, Cardo-Vila M et al (2006) Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol 176(10):5825–5832
Apte RS, Barreiro RA, Duh E, Volpert O, Ferguson TA (2004) Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Ophthalmol Vis Sci 45(12):4491–4497
Kampfer H, Brautigam L, Geisslinger G, Pfeilschifter J, Frank S (2003) Cyclooxygenase-1-coupled prostaglandin biosynthesis constitutes an essential prerequisite for skin repair. J Invest Dermatol 120(5):880–890
Kampfer H, Schmidt R, Geisslinger G, Pfeilschifter J, Frank S (2005) Wound inflammation in diabetic ob/ob mice: functional coupling of prostaglandin biosynthesis to cyclooxygenase-1 activity in diabetes-impaired wound healing. Diabetes 54(5):1543–1551
Streit M, Velasco P, Riccardi L et al (2000) Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J 19(13):3272–3282
Italiano JE Jr, Richardson JL, Patel-Hett S et al (2008) Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111(3):1227–1233
Acknowledgments
We thank Michelle Lazzaro and Yina Mo for their expert technical assistance. We thank Tissue Regenerative Technologies Inc. (Woodstock, GA) for the use of the DermaGold™ shockwave instrument. This work was supported by ONR work units 602236N.42237.W160.A0806 and 601153N.04508.5180.A0801.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defined a US Government work as a work prepared by a military service member or employees of the US Government as part of that person’s official duties. The views in this article are those of the authors and do not necessarily reflect the official policy of the Department of the Navy, Department of Defense nor the US Government. The experiments reported herein were conducted in compliance with the Animal welfare Act and in accordance with the principles set forth in the current edition of the Guide for Care and Use of Laboratory Animals, Institute for Laboratory Animal Resources, National Research Council, National Academy Press, 1996.
Stephen R. Zins and Mihret F. Amare contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zins, S.R., Amare, M.F., Tadaki, D.K. et al. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: effects of extracorporeal shock wave therapy. Angiogenesis 13, 293–304 (2010). https://doi.org/10.1007/s10456-010-9186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-010-9186-9