Abstract
The investigation of bioaerosols in the recent years has become more important both indoors and outdoors. Due to expanding of livestock farm capacity, raising attention is paid to this source of emissions. The objective of the present study was to estimate the distribution of bioaerosols in the animal husbandry and its surroundings. Over 2 fattening periods with different animal ages and weights, the main emphasis was put on the total concentrations of mesophilic bacteria and Staphylococcus spp., especially the species S. aureus. The bioaerosols were sucked in with an AGI-30 Impinger, and nasal and neck skinfold swab samples were collected supplementary from randomly selected pigs. From the first series of measurements, the total concentration of mesophilic bacteria was 6.2 × 105 cfu/m3 and dropped to 2.6 × 105 cfu/m3 with increasing growth of the swine. The concentration of Staphylococcus spp. was 8.8 × 104 cfu/m3 and decreased to 9.4 × 103 cfu/m3 by the end. In the empty barn, the total concentration of mesophilic bacteria was 9.1 × 104 cfu/m3 and for Staphylococcus spp., 1.1 × 104 cfu/m3. At the beginning of the second series of measurements, the total concentration of mesophilic bacteria was 6.6 × 104 cfu/m3 and dropped to 4.4 × 104 cfu/m3 with the increasing growth of the piglets. The concentrations of Staphylococcus spp. fluctuated along the second measurement series. The species Staphylococcus aureus was detected in nasal swabs of selected swine including MRSA. Temperature and humidity had no influence on the concentrations of mesophilic bacteria in the swine barn.
Similar content being viewed by others
1 Introduction
Animal husbandry has undergone a structural change toward large farms in recent years. The numbers of animals are increasing, while the number of farms decreases. In Germany, the number of animals increased by 5% from 2000 to 2016, but the farms reduced by 80% (Jungbluth et al. 2017). The bioaerosol emission increases due to the extension of the operating volume of the animal farms. Therefore, the emissions not only affect the air quality but also may impair the health of persons living near a livestock farm. In addition, there are unavoidable, unpleasant odors, which are considered a problem during building approval proceedings (Schiffmann et al. 1995; Liu et al. 2014).
Bioaerosol measurements are carried out at livestock farms and in the ambient air to assess health risks as well as environmental aspects. Occupational medical examinations have shown that people who are exposed to bioaerosols, such as bacteria, fungi and endotoxins, are more likely to suffer from health problems, respiratory diseases, infections or allergies (Herr et al. 1999; Radon and Nowak 2003). It is important to keep the emissions in the barn as low as possible in order to protect the health of farmers and animals and comply with animal welfare directives (VDI 4250 Part 1 2014).
Investigations inside animal housings compared with results from bioaerosol immission measurements in the surrounding of farms may help to assess the risk of potential negative health outcomes for the population in the right next neighborhood. An assessment of this risk factor is usually done by indicator parameters that characterize the emission of the pig farm buildings. The VDI standard specifies the emission factors for the parameter’s staphylococci in particular S. aureus, which occurs due to antibiotic resistance such as the methicillin-resistant S. aureus (MRSA) and intestinal enterococci, as these can act as potential pathogens in humans and animals (VDI 4255 Part 42017). Friese et al. (2012) and Schulz et al. (2012) reported the occurrence of MRSA in the exhaust air of swine farms and confirmed its colonization in animals’ noses. Previous studies reported that the bacterial concentrations in the barns increased with the increase in the weight and size of the animals (Chang et al. 2001; Sowiak et al. 2011; Kim and Ko 2019). The present study objectives had been planned to test this expectation in a swine barn in Styria, Austria.
1.1 Aim of the study
The present study was conducted to measure bioaerosols in pig barns within 2 separate fattening periods in order to determine the total concentrations of mesophilic bacteria with the focus on Staphylococcus spp. In addition, swab samples from nasal and neck skinfold of selected pigs were examined to detect S. aureus colonization.
2 Material and methods
2.1 The description of the pig barn
The barn is divided into 3 separate compartments with a size of 112 m2 for 138 animals. Each compartment includes 6 bays with 23 animals each. The fattening pigs are kept on a slit floor, and the ventilation is carried out through a porite ceiling with fresh air supply from the south-facing eave. In the selected swine barn, the 12-week-old piglets were housed in with a weight of 30–40 kg and kept in the compartments until they reached a slaughter weight of around 110 kg after a fattening period of 16 weeks. The feeding is biphasic depending on the age of the animals. After emptying, the barn was cleaned and disinfected to be prepared for the upcoming fattening period.
2.2 Bioaerosol measurements and animal sampling
Ten measurements in 2 series were conducted in one of the 3 compartments of the swine barn, and the first five M1–M5 (series I) took place in November and December 2018 and the second five M6–M10 (series II) in February and March 2019. The samples were collected once a week. Air temperature, humidity, sampling time, number and weight of the pigs were documented. In this study, 2 different swine herds were investigated in series I and series II measurements.
For each measurement, the AGI-30 Impinger (Ace Glass Inc., Vineland, USA) with a flow rate of 12.5 L/min (cutoff diameter: 0.31 µm) was applied for 30 min. The device was filled with 30 mL of phosphate-buffered saline (PBS) solution according to VDI 4257 Part 2 (2011) as a collecting medium and was set up approximately one meter above the floor in the barn (Fig. 1). The samples were transported in a sterile condition for the investigation process.
In both the first and second series of measurements, swab samples were taken from nasal and neck skinfold of 6 randomly selected swine (S1–S6) using sterile COPAN Transystem® cotton swabs. The microbiological examinations of the samples were processed within 12 h.
2.3 Cultivation and identification of the microorganisms
Serial dilutions of 100–10−3 were made from PBS collected samples by AGI-30 Impinger, and 100 or 500 µL was cultivated on specific agar media according to the instructions of the VDI guideline (VDI 4253 Part 3 2006). Subsequently, the agar plates were incubated at 37 °C for 48 h. Tryptic Soy Agar (TSA) was used to determine the total concentrations of mesophilic bacteria, and the colony-forming units per m3 (cfu/m3) of air were calculated.
For calculation of indicator parameters as staphylococci, aerococci and enterococci, the selective media are listed in Table 1.
For isolation of S. aureus from the 6 nasal and neck skinfold of swine, samples were streaked directly onto MAN and chromID™ S. aureus Elite (SAIDE) agar. Based on morphological criteria, the colonies were subcultured on COL agar.
Subsequently, the subcultures were qualitatively examined by means of VITEK® MS (bioMérieux, France), a MALDI-TOF mass spectrometry system. All identifications displaying a single result with a confidence value of 99.9% were considered acceptable for VITEK MS. Isolates yielding a single result without acceptable confidence level or multiple results or “no identification” results were retested (Neumeister et al. 2009; Kärpänoja et al. 2014). The retesting of bacterial identification was done using 16SrDNA PCR and comparing sequences with those available in the GenBank, EMBL and DJB databases using the gapped BLASTN 2.10.1 program through the National Center for Biotechnology Information server (Relman 1993; Altschul et al. 1997).
2.4 Antibiotic resistance testing and spa typing
The S. aureus identified by MALDI-TOF–MS in the samples from nasal and neck skinfold swabs of selected pigs were subjected to antibiotic resistance testing according to EUCAST V9.0, 2019 guidelines. The testing for susceptibility of bacterial strains to antibiotics was carried out by means of the agar diffusion test from BD BBL™ Sensi-Disc™ (BD, USA). The tested antibiotics were penicillin (P), cefoxitin (FOX), tetracycline (TE), clindamycin (CC), erythromycin (E), norfloxacin (NOR), mupirocin (MUP), linezolid (LZD), rifampicin (RA), fusidic acid (FA), sulfamethoxazole and trimethoprim (SXT), gentamicin (GM) (Mutschler et al. 2012; Fritsche 2016).
For further characterization of S. aureus isolates, spa typing was performed as described by Zhang et al. (2005) and Ruppitsch et al. (2006).
3 Results
Two series of measurements were done (series I, series II). The first one (M1–M5) took place at the end of a fattening period; in the second measurement series (M6–M10), the stable air was investigated at the beginning of a fattening period.
3.1 Barn occupancy during the measurement series I and II
In series I (M1–M5), the swine steadily gained weight and the number of animals decreased in the fact that some animals had already reached the slaughter weight and were taken away (Table 2). M5 was done in the empty barn, but cleaning and draining of liquid manure had not yet taken place. In series I, the humidity was around 60% at temperatures between 20 °C and 21 °C. In series II, the temperature was between 24 °C and 25 °C and the humidity reached 60% in the barn.
3.2 Quantitative analysis
The total concentrations of the mesophilic bacteria, Staphylococcus spp. and Aerococcus spp. of series I and II, are listed in Table 3. In the first series of measurements, a higher number of different genera were found than in the second series of measurements, whereas Enterococcus spp. could only be detected in the second series. Enterococci is not shown in the table or in the figures.
3.2.1 Series I measurements
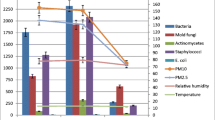
The concentrations of mesophilic bacteria decreased from 6.2 × 105 (M1) to 2.6 × 105 cfu/m3 (M3) during series I as the weight of the animals increased and then rose again in the M4 to 105 cfu/m3. M5 was done in the empty uncleaned barn with total concentrations of mesophilic bacteria of 9.1 × 104 cfu/m3. The concentrations of Staphylococcus spp. decreased from 8.8 × 104 (M1) to 9.4 × 103 cfu/m3 (M3) with the increasing weight of the animals. At M4, the concentrations of Staphylococcus spp. increased again to 3.1 × 104 cfu/m3. The Staphylococcus spp. achieved a concentration of 1.1 × 104 cfu/m3 at M5. The concentrations of Aerococcus spp. decreased from 3.3 × 105 (M1) to 4.1 × 104 cfu/m3 (M2) as the weight of the animals increased. The concentrations increased at M3 and M4 from 1.2 × 105 to 4.2 × 105 cfu/m3. The last measurement M5 in the empty barn shows concentrations of Aerococcus spp. of 1.6 × 104 cfu/m3. Standard error of measurements of mesophilic bacteria concentrations is also shown in Fig. 2.
3.2.2 Series II measurements
The total concentrations of mesophilic bacteria at the beginning of the measurements increased from 6.6 × 104 (M6) to 1.1 × 105 cfu/m3 (M7) and then dropped to 2.7 × 104 cfu/m3 (M9) during the following 2 measurements. At M10, the total concentrations of mesophilic bacteria increased again to 4.4 × 104 cfu/m3. The concentrations of Staphylococcus spp. show fluctuation, increased from M6 to M7 and dropped at M8 and then rose again within the last 2 measurements. The concentrations of Aerococcus spp. show fluctuations; the highest concentration was 1.1 × 105 cfu/m3 at M7, and the lowest was 2.3 × 104 cfu/m3 at M8. Standard error of measurements of mesophilic bacteria concentrations is also shown in Fig. 3.
3.3 Qualitative analysis
One hundred and sixty-six strains were identified by MALDI-TOF–MS and 16SrDNA PCR of which 94 strains were identified as Staphylococcus species.
3.3.1 Series I identification
Aerococcus viridans and S. pasteuri were the most common identified mesophilic bacterial species (n = 103) in the barn. No S. aureus species have been detected in the pigpen air (Fig. 4).
3.3.2 Series II identification
The largest proportion of investigated mesophilic bacterial colonies (n = 63) counted as A. viridans, S. pasteuri and S. cohnii. Additionally, intestinal enterococci (Enterococcus hirae) were detected in 8% (5 isolates), and no S. aureus species were found in the air of the piglet’s barn (Fig. 5).
Table 4 lists the mesophilic bacterial genera including the respective number of identified species of the 2 measurement periods, series I and II.
3.4 Swine nasal/neck skinfold swabs
In series I, S. aureus colonization was detected in the nose of one adult swine (S4), whereas S5 and S6 were tested negative. The spa typing revealed in a spa type t034 and resistance testing showed a methicillin-sensitive S. aureus (MSSA) with resistance to penicillin and tetracycline. In series II, S. aureus colonization was determined in the noses of 2 piglets (S1, S2) and in the neck skinfold of a third animal (S3). The result of spa typing shows that S1 and S2 were both carriers of spa type t011. Antibiotic resistance testing resulted in a MRSA S. aureus in S1 and a MSSA in animal S2. For S3, also spa type t011 was identified in the swine neck skinfold as MSSA. The 3 isolates (S1–S3) revealed resistance to penicillin, tetracycline, clindamycin and erythromycin. The assessment of antibiotic resistance is described in Table 5.
4 Discussion
Contrary to the expectation of the initial hypothesis of the present study that mesophilic bacterial concentration increases with increasing weight and size of the pigs, there was no such continuous increase seen in the investigated barn. In order to explain this fluctuation of mesophilic bacteria concentrations in the barn air, other factors such as measurements after daily feeding sessions, farm keeper work in the barn or pig selection for slaughtering could have had an impact on the results. Kim and Ko (2019) performed measurements in forced ventilated pig farms with a 6-stage cascade impactor and calculated a mean concentration of 1.2 × 104 cfu/m3. Their findings showed that the total concentration of mesophilic bacteria increased with the size and weight of the pigs and there was no fluctuation like we recorded in the present study. Sowiak et al. (2011) reported that the concentrations of microorganisms were significantly higher in case of small herds, the type of bedding system, manual feed distribution and natural ventilation of barns. Chang et al. (2001) investigated pigpens and found similar concentrations of mesophilic bacteria of 105 cfu/m3 at the end of the fattening period. By comparing different bioaerosol sampling methods, it was confirmed that the AGI-30 Impinger was the sampling device of choice for viable bacteria in pigpens (Thorne et al. 1992).
Due to the high level of liquid manure which was kept over the entire fattening period under slit floor, mesophilic bacteria were also detected in the uncleaned empty barn measurement in series I (M5). In the air of emptied and cleaned barns, the concentrations of mesophilic bacteria were lower than of 1.8 × 103 cfu/m3 (Martin et al. 1996; Bilić et al. 2000).
The total concentrations of mesophilic bacteria and Staphylococcus spp. in measurement series I of the hogs were one order of magnitude higher than that of the piglets in series II. This may be explained by the larger body surface of adult animals which may release more bacteria into the surrounding air. This indicates that the concentrations of Staphylococcus spp. in the barn air increase with increasing weight and size of the animals. Gärtner et al. (2017) reported a mean concentration of 2.0 × 105 cfu/m3 for Staphylococcus spp., whereas the present study measured a concentration of 8.8 × 104 cfu/m3 in series I. The emission levels of Staphylococcus spp. were higher in the exhaust air of forced ventilation farms than inside the barn air. Other studies focused on the detection of S. aureus in the exhaust air of pig farms but did not mention the total number of the genus Staphylococcus (Friese et al. 2012).
At present time, Aerococcus spp. are not considered as indicator parameters for emissions from pig farms. However, in the present study, aerococci (Aerococcus viridans) were the most frequently identified species besides staphylococci. Martin et al. (2007) and White et al. (2019) also identified A. viridans in the stable dust as one of the most abundant species. Another study showed that overall skin and nasal microbiota was rich of Aerococcus species (Strube et al. 2018).
The qualitative results of the present study show that in both series of measurements S. pasteuri, S. cohnii subsp. cohnii and A. viridans were the most commonly detected mesophilic bacteria. These results are confirmed by Martin et al. (1996) and Rich (2005). Roque et al. (2016) identified S. cohnii subsp. urealyticus and S. aureus as the most common mesophilic bacteria in the air of pigpen using a cascade impactor. S. cohnii was one of the most frequently identified Staphylococcus species in this study as well as subspecies S. cohnii subsp. cohnii. Predicala et al. (2002) performed measurements in 2 different ventilated pig farms using impactor and filtration methods and found that staphylococci were 70% of total mesophilic bacteria in the air which is similar to the results of the present study. Kim (2017) reported that the predominant airborne mesophilic bacteria in swine houses were Staphylococcus spp., Micrococcus spp. and Brevibacillus spp. However, the study by Vestergaard et al. (2018) found that pig stables have significantly lower airborne mesophilic bacterial diversity than farmer’s homes.
In the present study, E. hirae was detected only in series II. Novais et al. (2013) detected Enterococcus spp. in samples collected at pig farms and Liu et al. (2018) suggested Enterococcus spp. as indicator bacteria for investigations of contaminated barn materials. Nevertheless, only a small percentage of the cultivable airborne Gram-negative bacteria survive in the environment (Zucker et al. 2000; Schmithausen et al. 2018).
In the present study, no S. aureus or MRSA strain was detected in the barn air, but identified spa types from the pig swab samples correspond to the results of Friese et al. (2012). In contrast, low prevalence of MRSA was found in the air of pig farming communities in Sri Lanka (Kalupahana et al. 2019). Some studies show increased spread of S. aureus and MRSA in the stable air with increasing herd size, as well as on the animals’ skin. On the contrary, the study of Madsen et al. (2018) obtained the highest concentrations of S. aureus and MRSA in a weaner piglet stable, during high-pressure cleaning of an empty stable, and the lowest were found in a stable with sick pigs. Angen et al. (2017) concluded that the nasal MRSA contamination level of human is positively correlated with the air level of MRSA and not to the physical contact with pigs.
According to the antibiotic resistance, all S. aureus strains of the swab samples were identified as MSSA and only one animal was MRSA carrier. The spa typing revealed spa types t034 and t011. The t034 is very common in pigs and exists as either methicillin-resistant S. aureus (MRSA) or methicillin-sensitive S. aureus (MSSA). The spa type t011 is considered as a typical swine MRSA. The nasal swabs of the present study correspond to t011 and t034, whereas Agersø et al. (2012) reported spa type of t011, t034, t1451, t2876, t2974. In the report of Davies (2015), 33 spa types were detected. In 4 pig slaughterhouses, Ivbule et al. (2017) identified 15 different spa types among all MRSA isolates.
The S. aureus strains tested in this study showed resistance to Penicillin, Tetracycline, Clindamycin and Erythromycin. In addition to the aforementioned resistances, Cefoxitin resistance indicates a MRSA strain (Fernandes et al. 2005). According to the Austrian Agency for Health and Food Safety (AGES), Tetracyclines are among the most frequently administered antibiotics in pig farming. Less than 0, 1 tonnes of Cefoxitin were used in 2017 and could therefore explain the rare resistance of the tested S. aureus strains in the present study (Fuchs and Fuchs 2018).
5 Conclusion
The present study provides quantitative and qualitative microbial information of bioaerosols in a pig barn during 2 fattening periods.
There was a fluctuation of mesophilic bacteria concentrations in the barn air. The concentrations of Staphylococcus spp. increased during pig growth. S. aureus was found in nasal and neck skinfold swap samples of pigs but not in the barn air, and only one was tested MRSA positive with spa type t011. In future, investigations should be done for more herd groups with different pig numbers over whole fattening periods in order to obtain more reliable results and to evaluate the fluctuations of microorganism concentrations in the barn air.
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10453-020-09684-2
References
Agersø, Y., Hasman, H., Cavaco, L. M., Pedersen, K., & Aarestrup, F. M. (2012). Study of methicillin resistant Staphylococcus aureus (MRSA) in Danish pigs at slaughter and in imported retail meat reveals a novel MRSA type in slaughter pigs. Veterinary Microbiology, 157(1–2), 246–250.
Altschul, X., Stephen, F., Thomas, L., Madden, X., Alejandro, A., Schäffer, X., et al. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Angen, Ø., Feld, L., Larsen, J., Rostgaard, K., Skov, R., Madsen, A. M., et al. (2017). Transmission of methicillin-resistant Staphylococcus aureus to human volunteers visiting a swine farm. Applied and Environmental Microbiology, 83(23), 1–10.
Bilić, V., Habrun, B., Barač, I., & Humski, A. (2000). Distribution Of airborne bacteria in swine housing facilities and their immediate environment. Archives of Industrial Hygiene and Toxicology, 51(2), 199–205.
Chang, C. W., Chung, H., Huang, C. F., & Su, H. J. J. (2001). Exposure of workers to airborne microorganisms in open-air swine houses. Applied and Environmental Microbiology, 67(1), 155–161.
Davies, P. (2015). Prevalence and characterization of Staphylococcus aureus in pigs in the USA. In Research report, Public health/worker safety, 1–10.
Fernandes, C. J., Fernandes, L. A., & Collignon, P. (2005). Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. Journal of Antimicrobial Chemotherapy, 55, 506–510.
Friese, A., Schulz, J., Hoehle, L., Fetsch, A., Tenhagen, B. A., Hartung, J., et al. (2012). Occurrence of MRSA in air and housing environment of pig barns. Veterinary Microbiology, 158, 129–135.
Fritsche, O. (2016). Mikrobiologie (pp. 1–336). Berlin: Springer-Verlag.
Fuchs, R., Fuchs, K. (2018). Bericht über den Vertrieb von Antibiotika in der Veterinärmedizin in Österreich 2013–2017. Österreichische Argentur für Gesundheit und Ernährungssicherheit GmbH (AGES) 1–27.
Gärtner, A., Geburek, F., Geiger, J., Gessner, A., Gladtke, D., Hebbinghaus, H., et al. (2017). Bioaerosole aus der Tierhaltung (p. 55). Nordrhein-Westfalen: LANUV Fachbericht 80.
Herr, C., Bittighofer, P. M., Bünger, J., Eikmann, T., Fischer, A. B., Grüner, C., et al. (1999). Wirkung von mikrobiellen Aerosolen auf den Menschen. Gefahrstoffe: Reinhaltung der Luft, 59(6), 229–240.
Ivbule, M., Miklaševičs, E., Čupāne, L., Bērzina, L., Bālinš, A., & Valdovska, A. (2017). Presence of methicillin-resistant Staphylococcus aureus in slaughterhouse environment, pigs, carcasses, and workers. Journal of Veterinary Research, 61, 267–277.
Jungbluth, T., Büscher, W., & Krause, M. (2017). Technik Tierhaltung (2nd ed., pp. 13–294). Stuttgart: Ulmer-Verlag.
Kalupahana, R. S., Duim, B., Verstappen, K. M., Gamage, C. D., Dissanayake, N., Ranatunga, L., et al. (2019). MRSA in pigs and the environment as a risk for employees in pig: Dense areas of Sri Lanka. Frontiers of Sustainable Food Systems, 3(25), 1–8.
Kärpänoja, P., Harju, I., Rantakokko-Jalava, K., Haanperä, M., & Sarkkinen, H. (2014). Evaluation of two matrix-assisted laser desorption ionization–time of flight mass spectrometry systems for identification of viridans group streptococci. European Journal Clinical Microbiology Infection Disease, 33, 779–788.
Kim, K. Y. (2017). Field investigation of airborne bacteria in swine house of South Korea. Journal of Dairy and Veterinary Sciences, 4(4), 1–2.
Kim, K. Y., & Ko, H. J. (2019). Indoor distribution characteristics of airborne bacteria in pig buildings as influenced by season and housing type. Asian-Australasian Journal of Animal Sciences, 32(5), 742–747.
Liu, M., Kemper, N., Volkmann, N., & Schulz, J. (2018). Resistance of Enterococcus spp. in dust from farm animal houses: A retrospective study. Frontiers in Microbiology, 9(3074), 1–12.
Liu, Z., Powers, W., & Mukhtar, S. (2014). A review of practices and technologies for odor control in swine production facilities. Applied Engineering in Agriculture, 30(3), 477–492.
Madsen, A. M., Kurdi, I., Feld, L., & Tendal, K. (2018). Airborne MRSA and total Staphylococcus aureus as associated with particles of different sizes on pig farms. Annals of Work Exposures and Health, 62(8), 966–977.
Martin, V., Vela, A. I., Gilbert, M., Cebolla, J., Goyache, J., Dominguez, L., et al. (2007). Characterization of Aerococcus viridans isolates from swine clinical specimens. Journal of Clinical Microbiology, 45(9), 3053–3057.
Martin, W. T., Zhang, Y., Willson, P., Archer, T. P., Kinahan, C., & Barber, E. M. (1996). Bacterial and fungal flora of dust deposits in a pig building. Occupational and Environmental Medicine, 53, 484–487.
Mutschler, E., Geisslinger, G., Kroemer, H. K., Ruth, P., & Schäfer-Korting, M. (2012). Mutschler Arzneimittelwirkungen: Lehrbuch der Pharmakologie und Toxikologie (p. 736). Stuttgart: Wissenschaftliche Verlagsgesellschaft.
Neumeister, B., Geiss, H., Braun, R., & Kimmig, P. (2009). Mikrobiologische Diagnostik: Bakteriologie—Mykologie—Virologie—Parasitologie (2nd ed., pp. 166–170). Stuttgart: Georg Thieme Verlag.
Novais, C., Freitas, A. R., Silveira, E., Antunes, P., Silva, R., Coque, T. M., et al. (2013). Spread of multidrug-resistant Enterococcus to animals and humans: An underestimated role for the pig farm environment. Journal of Antimicrobial Chemotherapy, 68, 2746–2754.
Predicala, B. Z., Urban, J. E., Maghirang, R. G., Jerez, S. B., & Goodband, R. D. (2002). Assessment of bioaerosols in swine barns by filtration and impaction. Current Microbiology, 44, 136–140.
Radon, K., & Nowak, D. (2003). Atemwegs- und Lungenerkrankungen in der Europäischen Landwirtschaft. Pneumologie, 57(8), 444–448.
Relman, D. A. (1993). Universal bacterial 16S rRNA amplification and sequencing. In D. H. Persing, T. F. Smith, F. C. Tenover, & T. J. White (Eds.), Diagnostic molecular microbiology: Principles and applications (pp. 489–495). Washington, DC: ASM Press.
Rich, M. (2005). Staphylococci in animals: Prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillin-resistant Staphylococcus aureus. British Journal of Biomedical Science, 62(2), 98–105.
Roque, K., Lim, G. D., Jo, J. H., Shin, K. M., Song, E. S., Gautam, R., et al. (2016). Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings. Journal Veterinary Science, 17(4), 531–538.
Ruppitsch, W., Indra, A., Stöger, A., Mayer, B., Stadlbauer, S., Wewalka, G., et al. (2006). Classifying spa types in complexes improves interpretation of typing results for methicillin-resistant Staphylococcus aureus. Journal of Clinical Microbiology, 44(7), 2442–2448.
Schiffmann, S. S., Sattely Miller, E. A., Suggs, M. S., & Graham, B. G. (1995). The effect of environmental odors emanating from commercial swine operations on the mood of nearby residents. Brain Research Bulletin, 37(4), 369–375.
Schmithausen, R. M., Schulze-Geisthoevel, S. V., Heinemann, C., Bierbaum, G., Exner, M., Petersen, B., et al. (2018). Reservoirs and transmission pathways of resistant indicator bacteria in the biotope pig stable and along the food Chain: A review from a one health perspective. Sustainability, 10(3967), 1–26.
Schulz, J., Friese, A., Klees, S., Tenhagen, B. A., Fetsch, A., Rösler, U., et al. (2012). Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Applied and Environmental Microbiology, 78(16), 5666–5671.
Sowiak, M., Bródka, K., Buczyńska, A., Cyprowski, M., Kozajda, A., Sobala, W., et al. (2011). An assessment of potential exposure to bioaerosols among swine farm workers with particular reference to airborne microorganisms in the respirable fraction under various breeding conditions. Aerobiologia, 28(2), 121–133.
Strube, M. L., Hansen, J. E., Rasmussen, S., & Pedersen, K. (2018). A detailed investigation of the porcine skin and nose microbiome using universal and Staphylococcus specific primers. Scientific Reports, 8(12751), 1–9.
Thorne, P. S., Kiekhaefer, M. S., Whitten, P., & Donham, K. J. (1992). Comparison of bioaerosol sampling methods in barns housing swine. Applied and Environmental Microbiology, 58(8), 2543–2551.
VDI 4250 Part 1. (2014). Bioaerosols and biological agents: Risk assessment of source-related ambient air measurements in the scope of environmental health—Effects of bioaerosol pollution on human health (pp. 1–23). Berlin: Beuth-Verlag.
VDI 4257 Part 2. (2011). Bioaerosols and biological agents: Emission measurement sampling of bioaerosols and sampling in liquids (pp. 1–60). Berlin: Beuth Verlag.
VDI 4253 Part 3. (2006). Measurement of airborne microorganisms and viruses in ambient air: Culture based method for the quantitative determination of bacteria in air—Method after separation in liquids (pp. 1–58). Berlin: Beuth-Verlag.
VDI 4255 Part 4. (2017). Bioaerosols and biological agents: Emission factors for pig husbandry (pp. 1–20). Berlin: Beuth-Verlag.
Vestergaard, D. V., Holst, G. J., Basinas, I., Elholm, G., Schlünssen, V., Linneberg, A., et al. (2018). Pig farmer’s homes harbor more diverse airborne bacterial communities than pig stables or suburban homes. Frontiers in Microbiology, 9(870), 1–14.
White, J. K., Nielsen, J. L., & Madsen, A. M. (2019). Microbial species and biodiversity in settling dust within and between pig farms. Environmental Research, 171, 558–567.
Zhang, K., McClure, J. A., Elsayed, S., Louie, T., & Conly, J. M. (2005). Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. Journal of Clinical Microbiology, 43(10), 5026–5033.
Zucker, B. A., Trojan, S., & Müller, W. (2000). Airborne gram-negative bacterial flora in animal houses. Journal Veterinary Medicine, Series B, 47, 37–46.
Acknowledgements
This study was financially supported by the Federal Ministry of Agriculture, Regions and Tourism (BMLRT) as part of the commissioned Research Project No. 101263 (short name: LUQUASTA) and as a collaborative project between the BMLRT and the Styrian Provincial Government.
Funding
Open access funding provided by Medical University of Graz.. Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Funds for this project were provided by the Austrian Federal Ministry of Sustainability and Tourism and the Styrian Provincial Government. None of the other authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Additional information
The original online version of this article was revised: The word “enterococci” was incorrectly published in three occurrences. And “to” was missed between “M4” and “105 cfu/m3” in “M4 to 105 cfu/m3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haas, D., Köck, S., Fritz, T. et al. Bioaerosol measurements over a fattening period in a pig barn focused on the presence of Staphylococcus spp.. Aerobiologia 37, 1–12 (2021). https://doi.org/10.1007/s10453-020-09658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-020-09658-4