Abstract
Benthic microalgae (BMA) biomass and community structure in freshwater lotic systems are often limited by inorganic nutrient availability. We examined nutrient limitation of benthic algae biomass and community structure using nutrient diffusing substrates in four rivers along a land use gradient in the Lake Biwa basin, Japan. Ambient in-stream nutrient concentrations were correlated to catchment land use, with the highest nitrogen and phosphorus concentrations being found in rivers draining more forested catchments. Nutrient limitation of primary producer biomass and nutrient-driven changes in community structure were evident in all four rivers, regardless of in-stream nutrient concentrations and surrounding land use. BMA biomass (measured as chlorophyll log-response ratio) exhibited the greatest nutrient limitation in rivers with higher in-stream nutrient concentrations. The relationship to catchment land use was less clear, with the highest nutrient limitation being observed in the catchment with moderate amounts of agricultural and forested land use. Nutrient additions resulted in a shift from dominance by Bacillariophyceae (diatoms) to a mixed Bacillariophyceae-Chlorophyceae (chlorophyte) community in all rivers and this shift was most pronounced in the forested catchments. Changes within the diatom community with nutrient additions were also observed, although the shifts in diatom community within a river in response to nutrient additions were much smaller than the differences in diatom community composition among rivers. Diatom taxa classified as highly motile increased with nutrient additions in all rivers. Our results suggest that primary producer community in rivers may be sensitive to nutrient inputs even in areas with elevated nutrient concentrations and catchments dominated by agricultural land use. There is likely widespread nutrient limitation in rivers of Japan, across both in-stream nutrient and land use gradients and any increases in nutrient loading will likely stimulate benthic algal growth. Our findings highlight the importance of looking at both biomass and species composition to assess ecosystem-level impacts of elevated nutrient levels.

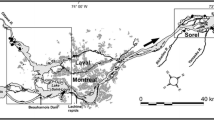
source: Maximilian Dörrbecker (Chumwa), CC BY-SA 3.0 < https://creativecommons.org/licenses/by-sa/3.0 > , via Wikimedia Commons; Shiga map source: Flappiefh, CC BY-SA 4.0 < https://creativecommons.org/licenses/by-sa/4.0 > , via Wikimedia Commons)









Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Aizaki M (1978) Seasonal changes in standing crop and production of periphyton in the Tamagawa river. Jpn J Ecol 28:123–134
Aizaki M (1979) Growth rates of microorganisms in a periphyton community. Jpn J Limnol 40:10–19
Aizali M, Sakamoto K (1988) Relationship between water quality and periphyton biomass in several streams in Japan. Verh Internat Verein Limnol 23:1511–1517
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol 35:257–284
American Public Health Association (APHA) (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Ateia M, Nasr M, Ikeda A, Okada H, Fujii M, Natsuike M, Yoshimura C (2016) Nonlinear relationship of near-bed velocity and growth of riverbed periphyton. Water. https://doi.org/10.3390/w8100461
Azim ME, Asaeda T (2005) Periphyton structure, diversity, and colonization. In Azim ME, Verdegem MCJ, van Dam AA, Beveridge MCM (eds) Periphyton: ecology, exploitation, and management, CABI, pp 15–33
Baekkelie KAE, Schneifer SC, Hagman CHC, Petrin Z (2017) Effects of flow events and nutrient addition on stream periphyton and macroinvertebrates: an experimental study using flumes. Knowl Manag Aquat. https://doi.org/10.1051/kmae/2017041
Beck WS, Rugenski AT, Poff NL (2017) Influence of experimental, environmental, and geographic factors on nutrient-diffusing substrate experiments in running waters. Freshw Biol 62:1667–1680
Beck WS, Markman DW, Olesky IA, Lafferty MH, Poff NL (2019) Seasonal shifts in the importance of bottom-up and top-down factors on steam periphyton community structure. Oikos 128:680–691
Bellinger EG, Sigee DC (2015) Freshwater algae identification, enumeration, and use as bioindicators 2nd edition. Wiley Blackwell, West Sussex, UK
Bennett EM, Carpenter SR, Caraco NE (2001) Human impact on erodible phosphorus and eutrophication: a global perspective. Bioscience 51:227–234
Biggs BJF, Kilroy C, Lowe RL (1998) Periphyton development in three valley segments of a New Zealand grassland river: test of a habitat matrix conceptual model within a catchment. Arch Hydrobiol 143:147–177
Blinn DW, Bailey PC (2001) Land-use influence on stream water quality and diatom communities in Victoria, Australia: a response to secondary salinization. Hydrobiologia 466:231–244
Bothwell ML (1989) Phosphorus limited growth dynamics of lotic periphytic diatom communities: areal biomass and cellular growth rate responses. Can J Fish Aquat Sci 46:1293–1301
Brett MT, Müller-Navarra DC (1997) The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw Biol 38:483–499
Brett MT, Müller-Navarra DC, Ballantyne AP, Ravet JL, Goldman CR (2006) Daphnia fatty acid composition reflects that of their diet. Limnol Oceanogr 51:2428–2437
Bridgeman TB, Chaffin JD, Kane DD, Conroy JD, Panek SE, Armenio PM (2011) From river to lake: phosphorus partitioning and algal community compositional changes in western Lake Erie. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2011.09.010
Burns CW, Brett MT, Schallenberg M (2011) A comparison of the trophic transfer of fatty acids in freshwater plankton by cladocerans and calanoid copepods. Freshw Biol 56:889–903
Camargo JA, Alonson A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32:831–849
Canham CD, Pace ML, Weathers KC, McNeil EW, Bedford BL, Murphy L, Quinn S (2012) Nitrogen deposition and lake nitrogen concentrations: a regional analysis of terrestrial controls and aquatic linkages. Ecosphere 3:1–16
Carr GM, Chambers PA, Morin A (2005) Periphyton, water quality and land use at multiple spatial scales in Alberta rivers. Can J Fish Aquat Sci 62:1309–1319
Cashman MJ, Wehr JD, Truhn K (2013) Elevated light and nutrients alter the nutritional quality of stream periphyton. Freshw Biol. https://doi.org/10.1111/fwb.12142
Chase JW, Benoy GA, Culp JM (2017) Combined effects of nutrient enrichment and inorganic sedimentation on benthic biota in an experimental stream system. Water Qual Res J 52(3):151–165
City of Kusatsu. 2019/20 (2nd year of Reiwa). 6th KUS City Comprehensive Plan. https://www.city.KUS.shiga.jp/shisei/seisaku/sogokeikaku/rokujisoukei/kikaku1201901.files/02-08.pdf
Cole JJ, Carpenter SR, Pace ML, Van de Bogert MC, Kitchell JL, Hodgson JR (2006) Differential support of lake food webs by three types of terrestrial organic carbon. Ecol Lett 9:558–568
Conroy JD, Kane DD, Culver DA (2008) Declining Lake Erie ecosystem health-evidence from a multi-year, lake-wide plankton study. In Munawar M. Heath RT (eds) Checking the pulse of Lake Erie, Michigan State University Press, Aquatic Ecosystem Health & Management Society, pp 369–408
Corkum LD (1996) Responses of chlorophyll a, organic matter, and macroinvertebrates to nutrient additions in rivers flowing through agricultural and forested land. Arch Hydrobiol 136:391–411
Correll D (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27:261–266
Cross WF, Benstead JP, Rosemond AD, Wallace JB (2003) Consumer-resource stoichiometry in detritus-based streams. Ecol Lett 6:721–732
Denicola DM (1996) Periphyton responses to temperature at different ecological levels. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, California, pp 149–181
Dent CL, Grimm NB (1999) Spatial heterogeneity of stream water nutrient concentrations over successional time. Ecology 80:2283–2298
Dodds WK (1991) Factors associated with dominance of the filamentous green alga Cladophora glomerata. Water Res 25:1325–1332
Dodds WK, Welch EB (2000) Establishing nutrient criteria in streams. J N Am Benthol Soc 19:186–196
Dodds WK, Biggs BJF, Lowe RL (1999) Photosynthesis-irradiance patterns in benthic microalgae: Variations as a function of assemblage thickness and community structure. J of Phycol 35:42–53
Dodds WK, Smith VH, Lohman K (2002) Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can J Fish Aquat Sci 59:865–874
Elser JJ, Bracken MES, Cleland EE, Gruner DW, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine, and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fairchild GW, Lowe RL, Richardson WB (1985) Algal periphyton growth on nutrient-diffusing substrates: an in situ bioassay. Ecology 66:465–472
Feminella JW, Hawkins CP (1995) Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. J N Amer Benthol Soc 14:465–509
Francoeur SN, Biggs BFJ, Smith RA, Lowe RL (1999) Nutrient limitation of algal biomass accrual in streams: seasonal patterns and a comparison of methods. J N Amer Benthol Soc 18:242–260
Francouer SN (2001) Meta-analysis of lotic nutrient amendment experiments: detecting and quantifying subtle responses. J N Amer Benthol Soc 20:358–368
Gillet ND, Pan Y, Asarian JE, Kann J (2016) Spatial and temporal variability of river periphyton below a hypereutrophic lake and a series of dams. Sci Total Environ 541:1382–1392
Gladyshev MI, Sushchik NN, Makhutova ON, Dubovskaya OP, Kravchuck ES, Kalachova GS, Khromechek EB (2010) Correlations between fatty acid composition of seston and zooplankton and effects of environmental parameters in a eutrophic Siberian reservoir. Limnologica 40:343–357
Goyette JO, Bennett EM, Maranger R (2018) Low buffering capacity and slow recovery of anthropogenic phosphorus pollution in watersheds. Nat Geosci 11:921–925
Greenwood JL, Rosemond AD (2005) Periphyton response to long-term nutrient enrichment in a shaded headwater stream. Can J Fish Aquat Sci 62:2033–2045
Griffith MB, Hill BH, Herlihy AT, Kaufmann PR (2002) Multivariate analysis of periphyton assemblages in relation to environmental gradients in colorado rocky mountain streams. J Phycol 38:83–95
Guo F, Kainz MJ, Sheldon F, Bunn SE (2016a) Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181:449–462
Guo F, Kainz MJ, Sheldon F, Bunn SE (2016b) The importance of high-quality algal food sources in stream food webs–current status and future perspectives. Freshw Biol 61:815–831
Hagy JD III, Houghton KA, Beddick DL Jr, James JB, Friedman SD, Devereux R (2020) Quantifying stream periphyton assemblage responses to nutrient amendments with a molecular approach. Freshw Sci 39:292–308
Hampton SE, McGowan S, Ozersky T, Virdis SGP, Tuong Thuy V, Spanbauer TL, Kraemer BM, Swann G, Mackay AW, Powers SM, Meyer MF, Labou SG, O’Reilly CM, DiCarlo M, Galloway AWE, Fritz SC (2018) Recent ecological change in ancient lakes. Limnol Oceanogr 63(5):2277–2304. https://doi.org/10.1002/lno.10938
Hawes I (1988) The seasonal dynamics of spirogyra in a shallow, maritime Antarctic lake. Polar Biol 8:429–437
Hayakawa A, Shimizu M, Woli KP, Kuramochi K, Hatano R (2006) Evaluating stream water quality through land use analysis in two grassland catchments: Impact of wetlands on stream nitrogen concentration. J Environ Qual 35(2):617–627. https://doi.org/10.2134/jeq2005.0343
Hill WR, Harvey BC (1990) Periphyton responses to higher trophic levels and light in a shaded stream. Can J Fish Aquat Sci 12:2307–2314
Hill WR, Knight AW (1988) Nutrient and light limitation of algae in two northern California streams. J Ecol 24:125–132
Hill BH, Herlihy AT, Kaufman PB, DeCelles SJ, Vander Borgh MA (2003) Assessment of streams of the eastern united states using a periphyton index of biotic integrity. Ecol Indic 2:325–338
Hill WR, Rinchard J, Czesny S (2011) Light, nutrients and the fatty acid composition of stream periphyton. Freshw Biol 56:1825–1836
Hirose H (1977) Illustrations of the Japanese fresh-water algae. Uchidarokuho Publishing Co., Ltd, Tokyo, Japan
Hoellein TJ, Arango CP, Zak Y (2011) Spatial variability in nutrient concentration and biofilm nutrient limitation in an urban watershed. Biogeochemistry 106:265–328
Hsieh CH, Ishikawa K, Sakai Y, Ishikawa T, Ichise S, Yamamoto Y, Kuo TC, Park HD, Yamamura N, Kumagi M (2010) Phytoplankton community reorganization driven by eutrophication and warming in Lake Biwa. Aquat Sci 72:467–483
Hsieh CH, Sakai Y, Ban S, Ishikawa K, Ishikawa T, Ichise S, Yamamura N, Kumagai M (2011) Eutrophication and warming effects on long-term variation of zooplankton in Lake Biwa. Biogeosciences 8:1383–1399
Iannino A, Vossahge ATL, Weitere M, Fink P (2020) Taxonomic shift over a phosphorus gradient affects the stoichiometry and fatty acid composition of stream periphyton. J of Phycol 56:1687–1695
Ichinose S, Wakabayashi T (2008) Japanese freshwater plankton illustrated handbook revised edition (in Japanese). Lake Biwa Environmental Research Institute, Otsu, Japan
Integrated Planning of Water Resource Quality (2000) Symposium on integration of basin management and applications to decision making and actual environment. Integrated planning of water resource quality. Kyoto University, Otsu, Japan, Department of Environmental Engineering, pp 103–105
Irvine RL, Jackson LJ (2006) Spatial variance of nutrient limitation of periphyton in montane, headwater streams (McLeod River, Alberta, Canada). Aquat Ecol 40:337–348
Ishikawa NF, Togashi H, Kato Y, Yoshimura M, Kohmatsu Y, Yoshimizu C, Ogawa NO, Ohte N, Tokuchi N, Ohkouchi N, Tayasu I (2016) Terrestrial-aquatic linkage in stream food webs along a forest chronosequence: multiisotopic evidence. Ecology 97:1146–1158
Johnson LT, Tank JL, Dodds WK (2009) The influence of land use on stream biofilm nutrient limitation across eight North American ecoregions. Can J Fish Aquat Sci 66:1081–1094
Jones NE (2010) Incorporating lakes within the river discontinuum: longitudinal changes in ecological characteristics in stream–lake networks. Can J Fish Aquat Sci 67:1350–1362
Katano I, Doi H (2019) Effects of Stream Grazers with Different Functional Traits on the Spatial Heterogeneity of Periphyton Mats. Peer J 7:e6747
Katano I, Doi H, Akiko Houki Y, Isobe TO (2007) Changes in periphyton abundance and community structure with the dispersal of a caddisfly grazer, Micrasema quadriloba. Limnology 8(3):219–226. https://doi.org/10.1007/s10201-007-0211-7
Kawanabe H (1996) Asian great lakes, especially Lake Biwa. Environ Biol Fish 47:219–223
Kazama S, Watanabe K (2018) Estimation of periphyton dynamics in a temperate catchment using a distributed nutrient-runoff model. Ecol Modell 367:1–9
Keck F, Lepori F (2012) Can we predict nutrient limitation in streams and rivers? Freshw Biol 57:1410–1421
Keithan ED, Lowe RL, DeYoe HR (1988) Benthic diatom distribution in a Pennsylvania stream: role of pH and nutrients. J Phycol 24:581–585
Kira T, Ide S, Fukada F, Nakamura M (2006) Lake Biwa: experiences and lessons learned brief. LakeNet: http://www.worldlakes.org/uploads/05_Lake_Biwa_27February2006.pdf
Kling GW, Kipphut GW, Miller MM, O’Brien J (2000) Integration of lakes and streams in a landscape perspective: the importance of material processing on spatial patterns and temporal coherence. Freshw Biol 43:477–497
Klose K, Cooper SD, Leydecker AD, Kreitler J (2012) Relationships among catchment land use and concentrations of nutrients, algae, and dissolved oxygen in a southern California river. Freshw Sci 31:908–927
Kramer K, Lange-Bertalot H (1986) Susswasserflora von mitteleuropa: bacillariophyceae, part 1. naviculaceae. Spektrum Akademischer Verlag, Heidelberg
Kramer K, Lange-Bertalot H (1988) Susswasserflora von mitteleuropa: bacillariophyceae, part 2. epithemiaceae, bacillariophyceae, surirellaceae. Spektrum Akademischer Verlag, Heidelberg
Kramer K, Lange-Bertalot H (1991a) Susswasserflora von mitteleuropa: bacillariophyceae, part 3. centrales, fragilariaceae, eunotiaceae, achnanthaceae. Spektrum Akademischer Verlag, Heidelberg
Kramer K, Lange-Bertalot H (1991b) Susswasserflora von mitteleuropa: bacillariophyceae, part 4. achnanthaceae. Spektrum Akademischer Verlag, Heidelberg
Kramer K, Lange-Bertalot H (2000) Susswasserflora von mitteleuropa: bacillariophyceae, part 5. Spektrum Akademischer Verlag, Heidelberg, English and French translation of keys
Lamberti GA, Gregory SV, Ashkenas LR, Li JL, Steinman AD, McIntire CD (1995) Influence of grazer type and abundance on plant-herbivore interactions in streams. Hydrobiologia 306:179–188
Lange K, Liess A, Piggott JJ, Townsend CR, Matthaei CD (2011) Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshw Biol 56:264–278
Larned ST (2010) A prospectus for periphyton: recent and future ecological research. J North Am Benthol Soc 29:182–206
Lobo EA, Katoh K, Aruga Y (1995) Response of epilithic diatom assemblages to water pollution in rivers in the Tokyo metropolitan area, Japan. Freshw Biol 34:191–204
Lohman K, Jones JR, Baysinger-Daniel C (1991) Experimental evidence for nitrogen limitation in a northern Ozark stream. J North Am Benthol Soc 10:14–23
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Lowe RL, Golladay SW, Webster JR (1986) Periphyton response to nutrient manipulation in streams draining clearcut and forested watersheds. J North Am Benthol Soc 5:221–229
Luecke C, MacKinnon P (2008) Landscape effects on growth of age-0 Arctic grayling in tundra streams. Trans Am Fish Soc 137:236–243
Marks JC, Lowe RL (1993) Interactive effects of nutrient availability and light levels on the periphyton composition of a large oligotrophic lake. Can J Fish Aquat Sci 50:1270–1278
Martin-Creuzburg D, von Elert E (2009) Good food versus bad food: the role of sterols and polyunsaturated fatty acids in determining growth and reproduction of Daphnia magna. Aquat Ecol 43:943–950
Martin-Creuzburg D, Von Elert E, Hoffmann KH (2008) Nutritional constraints at the cyanobacteria-Daphnia magna interface: the role of sterols. Limnol Oceanogr 53:456–468
Meals DW, Dressing SA, Davenport TE (2010) Lag time in water quality response to best management practices: a review. J Environ Qual 39(1):85–96. https://doi.org/10.2134/jeq2009.0108
Meybeck M (1998) Man and river interface: multiple impacts on water and particulates chemistry illustrated in the Seine River Basin. Hydrobiologia 373:1–20
Molloy JM (1992) Diatom communities along stream longitudinal gradients. Freshw Biol 28:59–69
Moreno-Peñarando R (2011) Japan’s urban agriculture: cultivating sustainability and well-being. https://unu.edu/publications/articles/japan-s-urban-agriculture-what-does-the-future-hold.html
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202–206
Munn MD, Osborne LL, Wilet MJ (1989) Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia 174:89–97
Nakano T, Tayasu I, Yamada Y, Hosono T, Igeta A, Hyodo F, Ando A, Saitoh Y, Tanaka T, Wada R, Yachi S (2008) Effects of agriculture on water quality of lake biwa tributaries, Japan. Sci Total Environ 389:132–148
Nitzsche KN, Shin K, Kato Y, Kamauchi H, Takano S, Tayasu I (2020) Magnesium and zinc stable isotopes as a new tool to understand Mg and Zn sources in stream food webs. Ecosphere 11:1–20
O’Brien PJ, Wehr JD (2010) Periphyton biomass and ecological stoichiometry in streams within and urban to rural land-use gradient. Hydrobiologia 657:89–105
Ogura A, Takeda K, Nakatsubo T (2009) Periphyton contribution to nitrogen dynamics in the discharge from a wastewater treatment plant. River Res Appl 25:229–235
Ohte N, Tayasu I, Kohzu A, Yoshimizu C, Osaka K, Makabe A, Koba K, Yoshida N, Nagata T (2010) Spatial distribution of nitrate sources of rivers in the Lake Biwa watershed, Japan: Controlling factors revealed by nitrogen and oxygen isotope values: nitrate sources of rivers in the lake biwa watersHED. Water Resour Res. https://doi.org/10.1029/2009WR007871
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Package ‘Vegan’. ISBN 0–387–95457–0
Okubo T (2012) Issues for reduction of pollution loads from point and nonpoint sources. In Kawanabe H, Nishino M, Maehata M (eds) Lake Biwa: interactions between nature and people Springer, Dordrecht, pp 433–440
Olesky IA, Baron JS, Beck WS (2021) Nutrients and warming alter mountain lake benthic algal structure and function. Freshw Sci 401:88–102
Ozersky T, Volkova EA, Bondarenko NA, Timoshikin OA, Malnik VV, Domysheva VM, Hampton SE (2018) Nutrient limitation of benthic algae in lake baikal, Russia. Freshw Sci. https://doi.org/10.1086/699408
Pan Y, Herlihy A, Kaufmann P, Wigington J, Van Sickle J, Moser T (2004) Linkages among land-use, water quality, physical habitat conditions and lotic diatom assemblages: a multi-spatial scale assessment. Hydrobiologia 515:59–73
Passy SI (2007) Differential cell size optimization strategies produce distinct diatom richness–body size relationships in stream benthos and plankton. J Ecol 95:745–754
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Ann Rev Ecol Evol Syst 32:333–365
Ponader KC, Charles DF, Belton TJ, Winter DM (2008) Total phosphorus inference models and indices for coastal plains streams based on benthic diatom assemblages from artificial substrates. Hydrobiologia 610:139–152
Powers SM, Zhang F (2016) Long-term accumulation and transport of anthropogenic phosphorus in three river basins. Nat Geosci. https://doi.org/10.1038/NGEO2693
Pringle CM (1987) Effects of water and substratum nutrient supplies on lotic periphyton growth: an integrated bioassay. Can J Fish Aquat Sci 44:619–629
Pringle CM (1990) Nutrient spatial heterogeneity: effects on community structure, physiognomy, and diversity of stream algae. Ecology 71:905–920
Pringle CM, Bowers JA (1984) An in situ substratum fertilization technique: diatom colonization on nutrient-enriched, sand substrata. Can J Fish Aquat Sci 41:1247–1251
Pringle CM, Paaby-Hansen P, Vaux PD, Goldman CR (1986) In situ nutrient assays of periphyton growth in a Lowland Costa Rican stream. Hydrobiologia 134:207–213
Reisinger AJ, Tank JL, Dee MM (2016) Regional and seasonal variation in nutrient limitation of river biofilms. Freshw Sci 35:474–489
Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D (2018) Package ‘MASS’. http://www.stats.ox.ac.uk/pub/MASS4/
Rosemarin AS (1983) Direct examination of growing filaments to determine phosphate growth kinetics in Cladophora glomerata (L.) Kutz and Stigeoclonium tenue (Agardh) Kutz. In: Wetzel DG (ed) Periphyton of freshwater ecosystems, Dr. W. Junk Publishers, Netherlands, pp 111–119
Rossiter A (2000) Lake Biwa as a topical ancient lake. Adv Ecol Res 31:571–598
Sadro S, Nelson CE, Melack JM (2012) The influence of landscape position and catchment characteristics on aquatic biogeochemistry in high-elevation lake-chains. Ecosystems 15:363–386
Sanderson BL, Coe HJ, Tran CD, Macneale KH, Harstad DL, Goodwin AB (2009) Nutrient limitation of periphyton in Idaho streams: results from nutrient diffusing substrate experiments. J North Am Benthol Soc 28:832–845
Scott JT, Lang DA, King RS, Doyle RD (2009) Nitrogen fixation and phosphatase activity in periphyton growing on nutrient diffusing substrata: evidence for differential nutrient limitation in stream periphyton. J North Am Benthol Soc 28:57–68
Smith VH (2003) Eutrophication of freshwater and marine ecosystems: a global problem. Environ Sci Pollut Res 10:126–139
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24:201–207
Smith VH, Joye SB, Howarth RW (2006) Eutrophication of freshwater and marine ecosystems. Limnol Oceanogr 51:351–800
Sonoda K, Yeakley JA, Walker CE (2001) Near-stream landuse effects on streamwater nutrient distribution in an urban watershed. J Am Water Resour Assoc 37:1517–1532
Squires LE, Ruthford SR, Brotherson DJ (1979) Algal response to a thermal effluent: study of a power station on the Provo River, Utah, USA. Hydrobiol 63:1011–1017
Steinman AD (1992) Does an increase in irradiance influence periphyton in a heavily-grazed woodland stream? Oecologia 91:163–170
Steinman AD, McIntire CD (1986) Effects of current velocity and light energy on the structure of periphyton assemblages in laboratory streams. J Phyc 22:352–361
Stelzer RS, Lamberti GA (2001) Effects of N: P ratio and total nutrient concentration on stream periphyton community structure, biomass, and elemental composition. Limnol Oceanogr 46:356–367
Stevenson RJ, Rier ST, Riseng CM, Schultz RE, Wiley MJ (2006) Comparing effects of nutrients on algal biomass in streams in two regions with different disturbance regimes and with applications for developing nutrient criteria. Hydrobiologia 561:149–165
Stockner JG, Shortreed KRS (1976) Autotrophic production in carnation Creek, a coastal rainforest stream Vancouver Island, British Columbia. J Fish Res Board Can 33:1533–1563
Tabuchi T, Yoshino K, Shimura M, Kuroda S, Ishikawa M, Yamaji E (1995) Relation between land use and nitrate concentration of outflow water from watersheds of agricultural and forest areas (Japanese with English summary). Jpn Soc Irrig Drain Reclam Eng 178:129–135
Taipale S, Strandberg U, peltomaa E, Galloway AWE, Ojala A, Brett MT, (2013) Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 streains of microalgae in 22 genera and in seven classes. Aquat Microb Ecol 71:165–178
Tanaka T. (1975) On the eutrophication of the river in or near urbanized areas (1) the effect of waste waters on the primary production in river bed (in Japanese) Water Purification and Liquid Wastes Treatment 16:345–349
Tank JL, Dodds WK (2003) Nutrient limitation of epilithic and epixylic biofilms in 10 North American streams. Freshw Biol 48:1031–1049
Tank JL, Reisinger AJ, Rosi E (2017) Nutrient limitation and uptake. In: Lamberti GA, Hauer FR (eds) Methods in Stream Ecology, vol 2. Ecosystem Function. Academic Press, London, pp 147–171
Taylor SL, Roberts SC, Walsh CJ, Hatt BE (2004) Catchment urbanisation and increased benthic algal biomass in streams: linking mechanisms to management. Freshw Biol 49:835–851
Tezuka Y, Watanabe Y, Hayashi H (1974) Changes in the standing crop of sessile microbes caused by organic pollution of the Tamagawa river. Jpn J Ecol 24:43–49
Timoshkin OA, Bondarenko NA, Volkova EA, Tomberg IV, Vishnyakov VS, Malnik VV (2014) Mass development of filamentous algae of genera Spirogyra and Stigeoclonium (Chlorophyta) in the coastal zone of southern Baikal. Hydrobiol J 50:13–23
Toda H, Uemura Y, Okino T, Kawanishi T, Kawashima H (2002) Use of nitrogen stable isotope ratio of periphyton for monitoring nitrogen sources in a river system. Water Sci and Technol 11–12:431–435
Tromboni F, Lourenço-Amorim C, Neres-Lima V, Thomas SA, Silva-Araújo M, Feijó-Lima R, Silva-Júnior EF, Heatherly T II, Moulton TP, Zandonà E (2019) Conversion of tropical forests to agriculture alters the accrual, stoichiometry, nutrient limitation, and taxonomic composition of stream periphyton. Int Rev Hydrobiol 104:116–126
Tsugeki NK, Urabe J, Hayami Y, Kuwae M, Nakanishi M (2010) Phytoplankton dynamics in Lake Biwa during the 20th century: complex responses to climate variation and changes in nutrient status. J Paleolimnol 44:69–83
Tyree MA, Carlisle DM, Spaulding SA (2020) Diatom enumeration method influences biological assessments of southeastern USA streams. Freshw Sci 39:183–195
Uemura Y (2012) Geomorphology of Lake Biwa and the surrounding region. In Kawanabe H, Nishino M, Maehata M (eds) Lake Biwa: interactions between nature and people Springer. Dordrecht, pp 3–8
Walker CE, Pan Y (2006) Using diatom assemblages to assess urban stream conditions. Hydrobiologia 561:179–189
Watanabe Y, Nishie K, Sakurai M (1975) Production of organic matter by sessile microbes in rivers (In Japanese). Journal of Water Waste 17:685–692
Watanabe T, Asai K, Houki A, Tanaka S, Hizuka T (1986) Saprophilous and eurysaprobic diatom taxa to organic water pollution and Diatom Assemblage Index (DAIpo). Diatom 2:23–73
Wehr J, Sheath R, Kociolek P (2002) Freshwater algae of North America. Academic Press, California, USA
Wickham H (2009) ggplot2 elegant graphics for data analysis. Springer, New York. https://doi.org/10.1007/978-0-387-98141-3
Winterbourn MJ (1990) Interactions among nutrients, algae and invertebrates in a New Zealand mountain stream. Freshw Biol 23:463–474
Wold AP, Hershey AE (1999) Spatial temporal variability of nutrient limitation in 6 north shore tributaries to Lake Superior. J North Am Benthol Soc 18:2–14
Woli KP, Hayakawa A, Kuramochi K, Hatano R (2008) Assessment of river water quality during snowmelt and base flow periods in two catchment areas with different land use. Environ Monit Assess 137:251–260
Yadav A, Kumar D, Singh RS, Pandey LK, Rai J (2018) Seasonal variations in response of periphytic algal community to nutrient enrichment in the river Ganga (Varanasi, India). Annales de Limnologie–International Journal of Limnology. https://doi.org/10.1051/limn/2018025
Yamada H, Nakamura F (2002) Effects of fine sediment deposition and channel works on periphyton biomass in the Makomanai River, northern Japan. River Res Appl 18:481–493
Yamamoto K (2014) GIS-based urbanization prediction model considering neighborhood relationship of the unit of the “block” in the outskirts of metropolitan area. Int J Geogr Inf Syst 6:330–344
Yoda M (2012) History of the relationship between people and Lake Biwa. In: Kawanabe Hiroya, Nishino Machiko, Maehata Masayoshi (eds) Lake Biwa: interactions between nature and people. Springer Netherlands, Dordrecht, pp 239–307. https://doi.org/10.1007/978-94-007-1783-1_4
Yoshikawa K (2004) On the progress of river restoration and the future view in Japan and Asia. In Geres D (ed) Third European Conference on River Restoration, Zagreb, Croatia, 17–21 May 2004. pp 43–55
Yoshimura C, Omura T, Furumai H, Tockner K (2005) Present state of rivers and streams in Japan. River Res Appl 21:93–112
Acknowledgements
This research was supported, in part, by a visiting researcher grant to C. L. Weilhoefer from Kyoto University Center for Ecological Research.
Funding
Partial financial support for C. L. Weilhoefer was received from Kyoto University Center for Ecological Research. No additional funding, grants, or support was received by other authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Ted Harris
Rights and permissions
About this article
Cite this article
Weilhoefer, C.L., Nakano, Si., Deb, S. et al. Nutrient limitation of primary production in rivers along a land use gradient in the Lake Biwa Basin, Shiga Prefecture, Japan. Aquat Ecol 56, 1177–1203 (2022). https://doi.org/10.1007/s10452-022-09971-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-022-09971-9