Abstract
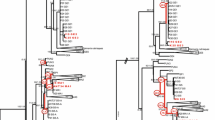
Diaphanosoma s.l., with 40+ described species, is the largest genus of the Sididae and the Ctenopoda, similar in many ways to the anomopod genus Daphnia. Here, we offer a c morphological evaluation of 33 species and contrast it with an analysis of the bar coding fragment of the cytochrome c oxidase subunit (COI) gene in order to gain insight into the taxonomy and phylogeny of the group. A search for structure in the genus based on micromorphological characters identified (1) the number of setae on the endopodite of P6 and (2) the relative length of the three apical setae of the exopodite of P6 as consistent markers, recovering two clades, the hexa-clade (with six endital setae) and the hepta-group (with seven setae). A third character is the armament of the posterior zone of the ventral margin of the valves, denticulated in the hexa-group, with fine filaments between the denticles in the hepta-group. Taxonomically, the two clades are assigned the rank of genera. Valid names for both are available in the published literature (Diaphanosoma Fischer s.s. and Neodiaphanosoma Paggi and Da Rocha, amended). A COI-based phylogenetic estimate leads to the same conclusion as morphology, but reveals cryptic speciation as well. COI reveals relatively small genetic distances within and between temperate climate species (e.g., in the D. birgei group) and large differences that form a cline from Australia (the terra typica) across southeast Asia in the tropical Neodiaphanosoma species D. excisum. African populations need more study, but probably represent a separate species. The entire group shows (macro)morphological stasis in the presence of molecular evolution but Neodiaphanosoma is evolving at a much faster rate than Diaphanosoma s.s. that is either a younger group. This hypothesis needs more testing, however. A biogeographic map recovers Neodiaphanosoma (containing slightly over 10 species) as restricted to the tropics, with limited penetration of the subtropics in the southern hemisphere. The more speciose Diaphanosoma group (up to 30 species) lives in the temperate and continental climate zone, with limited extension into subpolar zones, but considerable penetration of the subtropics and tropics. The subtropics, where both groups broadly overlap, is the zone with the highest species richness.









Similar content being viewed by others
Change history
19 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10452-021-09879-w
References
Alonso M (1996) Crustacea Branchiopoda. Fauna Iberica, vol 7. CSIC, Madrid
Costa FO, DeWaard JR, Boutillier J, Ratnasingham S, Dooh RT, Hajibabaei M, Hebert PDN (2007) Biological identifications through DNA barcodes: the case of the Crustacea. Can J Fish Aquat Sci 64:272–295
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Method 9(8):772
Dumont HJ, Negrea SV (2002) Branchiopoda. Guides to the identification of the microinvertebrates of the continental waters of the world, vol 19. Backhuys, Leiden
Elias- Gutierrez M, Martinez Jeronimo FM, Ivanova NV, Valdez-Moreno M, Hebert PDN (2008) DNA barcodes for Cladocera and Copepoda from Mexico and Guatemala, highlights and new discoveries. Zootaxa 1839:1–42
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3(5):294–299
Han B-P, Yin J, Lin X, Dumont HJ (2011) Why is Diaphanosoma (Crustacea: Ctenopoda) so common in the tropics? Influence of temperature and food on the population parameters of Diaphnaosoma dubum and a hypothesis on the nature of tropical cladocerans. Hydrobiologia 668:109–115
Hebert PDN, Ratnasingham S, DeWaard JR (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc B Biol Sci 270:96–99
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B Biol Sci 270:313–321
Huang CY, Zhou MX, Wang CC, Wei GJ (2001) Cooling of the South China Sea by the Toba eruption and correlation with other climate proxies—71,000 years ago. Geophys Res Lett 28:3915–3918
Jaume D (1991) The genus Diaphanosoma (Ctenopoda: Sididae) in Spain. Hydrobiologia 225:23–35
Katoh K, Standley DM (2013) MAFFT, multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Korovchinsky NM (1992) Sididae and Holopediidae (Crustacea: Daphniiformes). Guides to the identification of the microinvertebrates of the continental waters of the world, vol 3. SPB Academic, The Hague
Korovchinsky NM (1995) Redescription of Diaphanosoma volzi Stingelin, 1905 (Crustacea: Cladocera: Sididae) with remarks on comparative morphology, biology and geographical distribution. Hydrobiologia 315:189–201
Korovchinsky NM (2004a) Further notes on Diaphanosoma freyi Korovchinsky 2002 (Crustacea: Cladocera: Sididae). Arthropoda Sel 13(1–2):13–17
Korovchinsky NM (2004b) The Cladocera, order Ctenopoda, of the world (morphology, systematics, ecology, zoogeography). KMK Scientific Press, Moscow ([in Russian])
Korovchinsky NM (2006) The Cladocera (Branchiopoda) as a relict group. Zool J Linn Soc 147:109–124
Korovchinsky NM (2012) A new species of Diaphanosoma Fisher, 1850 (Crustacea: Cladocera: Sididae) from Japan. Limnology (2013) 14:13–18
Korovchinsky NM (2018) Cladocera: Ctenopoda: Families Sididae, Holopediidae & Pseudopenilidae (Branchiopoda: Cladocera). In: Dumont HJ (ed) Identification guides to the plankton and benthos of inland waters, vol 27. Backhuys Publishers, Leiden & Margraf Publishers, Weikersheim, pp 203
Korovchinsky NM, Sanoamuang L-o (2008) Overview of Sididae (Crustacea: Cladocera: Ctenopoda) of Northeast and East Thailand, with description of a new species of the genus Diaphanosoma. Zootaxa 1682:45–61
Korovchinsky NM, Timms BV (2001) A new species of the genus Diaphanosoma Fischer (Crustacea: Cladocera: Sididae) from Claypans in Western Australia. Proc Linn Soc N S W 133:1–10
Kotov AA (2007) Jurassic Cladocera (Crustacea, Branchiopoda) with a description of an extinct Mesozoic order. J Nat Hist 41:13–37
Kotov AA, Korovchinsky NM (2006) First record of fossil Mesozoic opoda) Ctenopoda (Crustacea, Ctenopoda). J Linn Soc 146:269–274
Kotov AA, Taylor DJ (2011) Mesozoic fossils (>145 Mya) suggest the antiquity of the subgenera of Daphnia and their coevolution with chaoborid predators. Evol Biol 11:129–138
Kotov AA, Van Damme K (2016) The fossil record of the Cladocera (Crustacea: Branchiopoda): evidence and hypotheses. Earth Sci Rev 163:162–189
Paggi JC, Rocha CEF (1999) Neodiaphanosoma, a new genus of Sididae (Branchiopoda, Ctenopoda) with description of N. bergamini sp. n. and comments on N. volzi (Stingelin, 1905). Hydrobiologia 387:5–19
Prosser S, Martínez-Arce A, Elías-Gutiérrez M (2013) A new set of primers for COI amplification from freshwater microcrustaceans. Mol Ecol Resour 13:1151–1155. https://doi.org/10.1111/1755-0998.12132
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Smirnov NN (1970) Cladocera (Crustacea) of Permian deposits from Eastern Kazakhstan. Paleontol Zh 3:95–100
Smirnov NN (1992) Mesozoic Anomopoda (Crustacea) from Mongolia. Zool J Linn Soc 104:97–116
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA
Xia X, Xie Z, Salemi M, Chen L, Wang Y (2001) An index of substitution saturation and its application. Mol Phylogenet Evol 26:1–7
Xiang X-F, Ji C-H, Shen S-Z, Gu G-L, Xu L, Han B-P, Kotov AA, Dumont HJ (2015) Annotated check-list of chinese Cladocera (Crustacea: Branchiopoda). Part 1. Haplopoda, Ctenopoda, Onychopoda, Anomopoda (families Daphniidae, Moinidae, Bosminidae, Ilyocryptidae). Zootaxa 3904:27
Acknowledgements
We dedicate this paper to the memory of Ramesh Gulati. Although he was a Daphnia man, he was interested in other cladocerans like Ceriodaphnia and Diaphanosoma as well. Although he was not a taxonomist, he insisted on using the correct name for the animals he worked with, acknowledging that a species not only had a specific morphology but a specific ecology also. The pioneer of this study was Mrs. Chen Hua, whose M Sci thesis contains the seeds of the morphological work required to study the characters used in this paper. But without adequate material, such a study would be futile. The godfather of ctenopod studies, Dr. Nikolai M. Korovchinsky from Moscow, deserves special thanks for donating a collection of not less than 24 species from all continents. Dr. Russell Shiel from Adelaide, Australia, and Dr. Marcelo Silva-Briano from Aguascalientes, Mexico, offered species from their local faunas and are thanked for their generous help. A special word of thanks to Prof. Larelle Fabbro of the CQ University at Rockhampton, Australia, for her repeated efforts to provide us with D. excisum from Sars’ type locality. She managed to find the species in between droughts and floods. Dr. John Beaver and Dr. John Havel (USA) provided samples from the East coast of the USA; Dr. Rey Donne S Papa and Mr. Eric Zeus C. Rizo traveled with us to the field in Luzon, Philippines. Mr. Sinyo, environmentalist and tourist guide, provided excellent guidance on the island of Sulawesi, sampling the ancient Malili lakes, but also smaller, shallow water bodies. Mr. Y L Gu accompanied one of us (HJD) during a collecting trip to Lake Toba, Sumatra. E. Nelson dos Santos Silva (Manaus) kindly provided samples from Amazonian varzea lakes. Dr. J C Paggi (Santo Tome, Argentina) kindly checked the distribution map for the hexa-species and added several records from South America that had been overlooked by us. The authors thank the Brazilian Long Term Studies Program (PELD, Site 4), National Council for Scientific and Technological Development (CNPq) for logistic support and Lorenna C. Cruz, Marcela M. Ribeiro and Evanguedes Kalapothakis for help with the DNA sequencing of Diaphanosoma brevireme and D. spinulosum in Belo Horizonte, Brasil.
Funding
This research was supported by Grants from the National Science Foundation of China (NSFC) (No: 31670460 to BP Han and No. 31901098 to Ping Liu) and a leading talent scientist of Guangdong Province, China, to HJ Dumont which permitted HJD not only to travel around China, but also to do field work in the adjacent south Asian countries.
Author information
Authors and Affiliations
Contributions
HJD and B-PH designed the research, HJD and HC worked on taxonomy, HC, FFG, CD, PL, LX, SX and AV did molecular analysis, and L-OS, ACR and ME-G supported taxonomy and phylogenetic analysis.
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dumont, H.J., Han, BP., Guo, F.F. et al. Toward a phylogeny and biogeography of Diaphanosoma (Crustacea: Cladocera). Aquat Ecol 55, 1207–1222 (2021). https://doi.org/10.1007/s10452-020-09819-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09819-0