Abstract
Species distribution, ecology, and behaviour are shaped, amongst other things, by interspecific, antagonistic interactions, and this phenomenon is particularly noticeable among predators. We studied the spatial co-distribution of two top piscivorous bird species foraging on inland waters outside breeding season. We considered the hypothesis that goosanders, Mergus merganser, and great cormorants, Phalacrocorax carbo, avoid foraging in close proximity to each other. Data collected on five river-reservoir systems in the Western Carpathians (Poland and Slovakia) during two periods (2014–2015 and 2015–2016) showed that goosander numbers reduced significantly and their foraging areas changed when large flocks of cormorants arrived and began foraging. We also found that inter-flock distances were greatest between flocks of goosanders and cormorants, suggesting that the former species avoided the waters occupied by the latter. Distribution of flocks of both species was additionally determined by the location of foraging place in river-reservoir system, weather, and presence of other piscivorous birds (e.g. grebes) and raptors (e.g. eagles). Together with the results of research in adjacent Bohemia, this study suggests that competition between cormorants and goosanders may arise when bodies of water suitable for piscivorous foraging are scattered and limited in number, as in the mountainous areas of Central Europe.
Similar content being viewed by others
Introduction
Interspecific interactions amongst animals have substantial effects on species distribution, ecology, and behaviour. These phenomena have been widely studied in various animal groups (Schoener 1974, 1982), and many of the studies have described relationships between predatory species (e.g. Capizzi and Luiselli 1996; Goldsworthy et al. 2001; Tannerfeldt et al. 2002; Berger and Gese 2007; Bur et al. 2008; Amirowicz and Gwiazda 2012). The majority of studies of interspecific interactions in birds have primarily been concerned with the breeding period, when competition for resources is highest (e.g. Kostrzewa 1991; Camphuysen 1995; Chakarov and Krüger 2010; Sergio et al. 2007). Relatively few studies have considered interactions in the post-breeding period or outside the breeding season (e.g. Blázquez et al. 2009; Paprocki et al. 2014) and only a few of them have concerned piscivorous species (e.g. Morkūnė 2011; Orben et al. 2015; Wood and Stillman 2014). Piscivorous birds are interesting species in which to study interspecific interactions as they are apex predators on water bodies and usually display complex relations in multispecies guilds. Moreover, larger piscivores are also considered important competitors for fisheries (e.g. Marquiss and Carss 1994; Carpentier et al. 2003; Mous et al. 2003; Stempniewicz et al. 2003; Wilson et al. 2003; Harris et al. 2008; Östman et al. 2013; Manikowska-Ślepowrońska et al. 2016). Studies of this group are, therefore, important for at least two reasons. First, simply because they can improve our fundamental ecological and ethological understanding of use of space amongst piscivorous predators. Second, they can be used to address the economic impact of piscivores on angling and fisheries.
During migration and wintering several of the species of larger piscivorous birds present in Central Europe may be involved in interspecific interactions (including direct competition or avoidance) for foraging places (Žydelis and Kontautas 2008). The piscivorous guild includes raptors, herons, gulls, grebes, divers, mergansers, and cormorants. The latter four members of this guild share a common hunting behaviour, namely diving for fish, although they differ with respect to preferred prey size and depth and duration of underwater hunting (e.g. Sjöberg 1985, 1988; Wood and Hand 1985; Dirksen et al. 1995; Van Dobben 1995; Carrs et al. 2012). Among them, only goosander, Mergus merganser (Linnaeus, 1758), and great cormorant, Phalacrocorax carbo (Linnaeus, 1758), regularly form large and compact flocks (Cramp and Simmons 1977; Van Eerden and Voslamber 1995) on the inland waters of Central Europe, whereas others do it only occasionally [e.g. great crested grebe Podiceps cristatus (Linnaeus, 1758); Källander 2008].
Piscivorous birds with similar body size, diet, and flocking behaviour are a good model system in which to study interspecific competition. For this study, we selected populations of goosanders and cormorants present on freshwaters of the Western Carpathians during autumn, winter, and early spring in which to investigate whether and how these species interact with each other to distribute the available foraging places. We tested the hypothesis that in areas with limited access to water bodies, goosanders would decrease in number when larger flocks of cormorants arrived and stayed to hunt. We predicted that goosanders and cormorants avoid foraging in the same places, based on preliminary findings from programs monitoring wintering populations of both species in Czechia, Slovakia, and Poland (Chodkiewicz et al. 2013; Musil et al. 2015; Wilk et al. 2016).
Methods
Study area
We selected the freshwaters of the Western Carpathians as our study area, because there, especially during the winter, the water bodies available for piscivorous birds are scattered and limited in size. Moreover, in the Western Carpathians, the context of the relationship between the two species is very unusual. Only goosanders breed in the area, although the species only settled there quite recently (less than two decades ago; Kajtoch and Bobrek 2014). Over the past few years, the breeding population of goosanders in the Western Carpathians has increased significantly, resulting in an increase in goosander numbers on water bodies outside the breeding season (Musil et al. 2015). Thus they have become a regular species across the whole year and only during winter do numbers increase due to the arrival of wintering of birds from northern populations (probably of Baltic, Scandinavian, and Russian origin). On the other hand, cormorants do not breed in the area and are rare from late spring to late summer. They migrate in large flocks during autumn and early spring, but in winter they are present only on some ice-free water bodies.
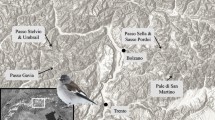
Data on the occurrence of both target species—goosanders and cormorants—and additional taxa that may be involved in predatory interactions (white-tailed eagle Haliaeetus albicilla (Linnaeus, 1758)) and by competition for food (large gulls such as mainly caspian gull Larus cachinnans (Pallas, 1811) and herring gull L. argentatus (Pontoppidan, 1763); grebes such as great crested grebe and red-necked grebe Podiceps grisegena (Boddaert, 1783), and divers such as red-throated diver Gavia stellata (Pontoppidan, 1763) and black-throated diver Gavia arctica (Linnaeus, 1758)) were collected in five river-reservoir systems in the Western Carpathians, three of which were in Poland (the Soła river with Żywiec reservoir of area 10.0 km2 [N49°53.3′, E19°12.3′; 340 m a.s.l.], the Raba river with Dobczyce reservoir of area 9.8 km2 [N49°52.1′, E20°2.5′; 270 m a.s.l.], the Dunajec river with Czchów reservoir of area 3.5 km2 [N49°48.9′, E20°39.2′; 230 m a.s.l.]) and two in Slovakia (the Hron river with Kozmálovce reservoir of area 0.7 km2 [N48°16.5′, E18°31.5′; 180 m a.s.l.] and the Slatina river with Môťová reservoir of area 0.6 km2 [N48°33.5′, E19°10′; 300 m a.s.l.) (Fig. 1).
Simplified map of the Western Carpathians presenting the localisation of river-reservoir systems in which piscivorous birds were inventoried. SZ Soła-Żywiec system, RD Raba-Dobczyce system, DC Dunajec-Czchów system, HK Hron-Kozmálovce system, SM Slatina-Môťova system, A country borders, B major rivers, C range of the Carpathians, D sampling sites
Bird inventories
Birds (cormorants, goosanders, grebes, divers, large gulls, and eagles) were counted during the middle of every month from September until March, in two periods: 2014–2015 and 2015–2016 (7 month of counts during each period).
Inventories were standardised into four sections to enable us to estimate the distribution and movement of bird flocks on river-reservoir systems of different sizes: (1) along river fragments 750–250 m above the backwater; (2) on reservoirs, counts from a point localised up to 1000 m below the backwater; (3) on reservoirs, counts from a point localised up to 1000 m above the dam; (4) along river fragments 0–500 m below the dam. In “Methods” section and “Results” section, counts were executed at a visibility distance of c. 500 m. In “Introduction” section and “Discussion” section, counts were executed by “walking” along a 500 m of the river channel. Each count in each section lasted for 15 min. The selection of sections was designed to cover a wide spectrum of the available freshwaters used by piscivores: man-made reservoirs on rivers (in two areas: close to the dam, where water often freezes during severe winters, and in the backwater where water is usually ice free throughout the winter) and river channels below and above reservoirs (the former are usually ice free, whereas the latter tend to freeze during cold periods of winter). Moreover, these sections also differed with respect to fish assemblages (running water species in rivers; stagnant water species in reservoirs) and foraging conditions (deep waters in the reservoirs; shallow but fast-running waters in the rivers).
Separate counts for each section and each flock were recorded on a special sheet. The approximate localisation of all goosanders and cormorants was noted on maps saved and printed from geoportals (http://mapy.geoportal.gov.pl/imap/ and https://www.geoportal.sk/sk/geoportal.html). Birds were observed and counted with the help of telescopes and binoculars, by experienced observers. Counts were estimated before midday, but usually after early morning, because in the river valleys and over reservoirs dense fog often limited visibility and hindered inventory taking and in order to reduce the risk of multiple counting of birds moving from sleepover to foraging areas after dusk. All counts were executed in good weather conditions (without precipitation or heavy wind and large waves) and only if visibility exceeded 500 m (usually if the observer could see the birds at a distance greater than 1 km).
Collection of variables
Several variables were noted during all counts. These variables were selected to describe the location where the inventory was taken, the duration of counts, and the weather conditions during the counts. These variables were as follows: (i) Site (location of river-reservoir system), (ii) Section (location of count within the system), (iii) Season (2014–2015 or 2015–2016), (iv) Temperature (average temperature during the count in °C), and (v) IceCover (estimated percentage of body of water covered by ice—estimated from simplified maps sketched by the observers). Next, total numbers of the following bird species during the count in the relevant section were noted: (i) Eagles, (ii) Gulls, (iii) Grebes, (iv) Goosanders, and (v) Cormorants. Divers were eventually excluded from the analyses as representatives were observed only occasionally (only five individuals during both periods on all systems).
In the case of goosanders and cormorants, all birds (individuals and flocks) were counted and their position mapped. Maps showing the approximate distribution of goosander and cormorant flocks were then used to calculate the shortest straight-line intraspecific and interspecific distances (to c. 10-m accuracy) between flocks.
Data analysis
Numbers of both species during both study periods were analysed and compared using the Chi-square test to evaluate migration and wintering patterns. Next, we calculated Spearman’s rank correlations between raw numbers of goosanders and cormorants in each survey (separate correlations were calculated for each section of all reservoirs for every monthly count). Correlations were calculated for two datasets: (1) all birds noted and (2) cases when at least one species was present as a flock of at least 10 individuals. The Mann–Whitney U test was used for several comparisons of flock size: cormorants alone versus cormorants in the presence of less than 10 goosanders; in the presence of less than 10 goosanders versus cormorants in the presence of more than 10 goosanders; goosanders alone versus goosanders in the presence of less than 10 cormorants; goosanders in the presence of less than 10 cormorants versus goosanders in the presence of more than 10 cormorants.
Intraspecific and interspecific distances for all datasets and for cases where only both species occurred simultaneously were compared using the Mann–Whitey U test (paired comparisons) and Kruskall–Wallis ANOVA (comparison of all conditions). Next, the ratio of distances within cormorants, within goosanders, and between cormorants and goosanders was compared as above.
Spearman’s rank correlations between all pairs of examined explanatory variables were assessed. Only two variables (Temperature and IceCover) were correlated, significantly but moderately (ρ = −0.46). Therefore, all variables were included in models of determinants of goosander and cormorant abundance. Abundance of Cormorants was included as a potential explanatory variable in models of goosander abundance and vice versa. After confirming that all model residuals met the assumptions of generalised linear models (GLMs), a set of competitive models was built based on the Poisson distribution. The performance of the models was evaluated in two steps: (i) univariate models were evaluated using Wald statistics and (ii) multivariate models were evaluated using Akaike’s information criterion (AIC), delta AIC (ΔAIC), and AIC weights (AIC w). Following Arnold (2010), we treated parameters which did not improve the AIC of a model by more than 2.0 as uninformative. All analyses were executed in Statistical v. 11 software (StatSoft Inc. 2001).
Results
Migration and wintering
In total (on all examined river-reservoir systems) we counted 1340 cormorants in the 2014–2015 period and roughly half that number (627 individuals) in the 2015–2016 period. In contrast, the total number of goosanders differed only marginally between the two periods: 825 and 730 individuals in 2014–2015 and 2015–2016, respectively. In comparison, the great crested grebe was found in much smaller but consistent numbers: 148 and 164 individuals in 2014–2015 and 2015–2016, respectively. There was clear variation in the relative abundance of cormorants and goosanders across the 2014–2015 period. Cormorants were more abundant during October and January–February, whereas goosanders were more abundant in December (Fig. 2). During the 2015–2016 period, there was no similarly clear pattern in relative species abundance. Once again cormorants were found in abundance in October and January, but in December both species were found in similarly high numbers (Fig. 2). Within each species, abundance differed between the two periods (cormorants: χ 2 = 168.2, df = 6, p < 0.001; goosanders: χ 2 = 30.1, df = 6, p < 0.001). Within each period the abundance of the two species differed (2014–2015: χ 2 = 299.5, df = 6, p < 0.001; 2015–2016: χ 2 = 112.5, df = 6, p < 0.001).
Co-distribution
In cases where only one species was present, the average flock size was twice as high for cormorants as for goosanders (Table 1A). When both species were noted during the count, cormorants were more numerous, especially in cases with cormorant flocks larger than 10 individuals (Table 1A). These differences were significant when the number of birds in flocks was compared in cases when competitor species was absent or in low number (below 10 individuals) and when competitor was in large number (above 10 individuals) (cormorants: Z = 3.54, p < 0.001; goosanders: Z = 2.53, p = 0.012). The numbers of each species were only negatively correlated (ρ = −0.67) in cases where at least one species was present as a flock of more than 10 individuals (Fig. 3).
In descriptive terms, the distance between goosander flocks was 1.7 times smaller than the distance between cormorant flocks, but the interspecific flock distance was greatest (Table 1B). These differences were significant always when the following comparisons regarding distances were carried out—cormorants: cormorants to distances goosanders: goosanders (Z = 4.78, p < 0.001); cormorants: cormorants to cormorants: goosanders (Z = 5.67, p < 0.001); and goosanders: goosanders to cormorants: goosanders (Z = 2.39, p = 0.017), and also all combinations were compared (ANOVA χ 2 = 16.84, p < 0.001). Therefore, the ratio among the distances of flocks was the lowest for goosander: cormorant/cormorant: cormorant and similar for cormorant: cormorant/goosander: goosander and goosander: cormorant/goosander: goosander (Table 1C). These ratios were found to be significantly different only in comparisons between cormorant: cormorant/goosander: goosander to goosander: cormorant/cormorant: cormorant (Z = 2.19, p = 0.028) but not significant in other comparisons.
Model selection
Separate univariate models for each species showed that almost all the analysed variables were predictors of either cormorant or goosander abundance (Table 2) with exception of Temperature and IceCover in case of cormorants and Gulls, Eagles and Temperature in case of goosanders. This meant that for both species, models that contained all or almost all variables and components provided the best explanation of abundance (Table 3). Based on the principles described by Arnold (2010), the best model of cormorant abundance contained the following variables: Goosanders, Eagles, Gulls, Site, Section and Season, whereas the best model of goosander abundance included all variables except Eagles (Table 3).
Discussion
In their recent report on trends in the numbers of wintering cormorants and goosanders in Central Europe between 1991 and 2013 Musil et al. (2015) noted that “… negative trends of goosanders were recorded in larger rivers in Central Bohemia (Labe and Vltava), especially since the regular wintering of larger numbers of cormorants in the area. We assume the existence of competition between these two fish-eating species, especially in large rivers”. Our results generally corroborate this with respect to non-breeding populations of goosanders and cormorants on bodies of water in the Western Carpathians although the pattern was not as clear.
First, bird numbers varied across the 2014–2015 and 2015–2016 periods. Seasonal variability in numbers of migrating and wintering species is a well-known phenomenon (e.g. Berthold 1993; Rubolini et al. 2007) and reflects the duration and timing of migration (products of evolutionary adaptation), and ecological constraints (e.g. weather and environmental conditions that limit survival). Cormorant numbers were especially variable, both within the examined periods (with peak abundance during autumn and early spring migration) and between periods (probably reflecting conditions in areas north to the Carpathians—i.e. on waters around the Baltic coasts—which were less severe during 2015–2016, leading to lower numbers of birds coming to the south; Suter 1995). Variation in goosander numbers was much lower as this species is migratory in the north (Hansen 1976; Marquiss and Duncan 1994), but partially sedentary in Central Europe (Keller 2009; Kajtoch and Bobrek 2014). These results suggest that although weather conditions (e.g. temperature and ice cover) affected numbers of both species during counts, they were less important than, for instance, the location of water bodies with bird flocks.
The distribution of flocks of both species was dependent on the type of water (running and shallow on rivers; stagnant and deep in reservoirs), which may be linked to prey fish availability. Both species can forage on medium and large rivers and on reservoirs, which are present in the Carpathians. There are probably some differences in the two species’ foraging strategies for example with respect to preferred depth of water for hunting, preference for stagnant or running water, but these differences are probably only detectable during breeding season (when cormorants usually hunt on larger, deeper, stagnant waters, including coastal waters, whilst goosanders often forage on shallow, running inland waters) (Ross 1977; Sjöberg 1985, 1988; Wood and Hand 1985; Marquiss and Carss 1994; Dirksen et al. 1995; Van Dobben 1995; Gibbons and Withers 2006; Carss et al. 2012). Outside the breeding season, there are probably no differences in foraging habits, as during migration and wintering both species forage on various waters, from the open sea to inland rivers, lakes, and ponds (Gilissen et al. 2002; Musil et al. 2011). Of course weather and type of water affected the distribution and numbers of both species, but these factors did not explain the local distribution of flocks.
The data we collected showed that goosanders and cormorants avoided each other outside the breeding season but this was not obvious. Analysis of the co-location of the two species suggests that goosander numbers decreased significantly when cormorants were present in large numbers. The converse relationship was not as clear, although on some bodies of water where goosanders dominated cormorants were less numerous. This pattern was most obvious in cases where flocks of at least 10 individuals were noted, when there was a significant negative correlation between goosander and cormorant numbers. The average size of cormorant flocks was about 1.5 times smaller when goosanders were numerous, but goosander flocks were about 2.5 times smaller when cormorants were numerous. The same pattern was found approximate in inter-flock distances; goosander–goosander flock distance was smallest (average = 100 m), cormorant–cormorant flock distance was larger (average = 170 m), and the interspecies flock distance was largest (average = 210 m). This pattern shows that, in sympatry, both species tried to occupy distant foraging locations outside the breeding season. Any explanation of this phenomenon is necessarily somewhat speculative owing to the limitations of our observational data. In view of the ecology of the two species, especially their feeding preferences (Sjöberg 1985, 1988; Wood and Hand 1985; Dirksen et al. 1995; Van Dobben 1995; Carss et al. 2012), the most probable explanation for their avoidance of proximity is competition for food, but this remains speculative as we have no direct evidence (we were unable to estimate abundance of fish on such a varied set of water bodies, estimating fish abundance concurrently with counting of birds would have been particularly problematic). Both goosanders and cormorants feed on various species and sizes of fish (Cramp and Simmons 1977). In the same habitat, their prey can be similar, so their feeding niches overlap (Sjöberg 1985, 1988; Wood and Hand 1985; Dirksen et al. 1995; Van Dobben 1995; Carss et al. 2012). The fact that they have common prey probably forces these species to hunt in different places; however, the avoidance is uneven and the goosander should be considered the subordinate species, forced to look for other foraging places when cormorants are present on the same body of water. This was especially noticeable when cormorants were numerous and occupied some part of a reservoir. In such cases, goosanders were usually noted as single individuals, with flocks found on adjacent rivers. This avoidance is probably not related to direct competition, because during counts the observers did not note any instances of cormorants chasing goosanders out of a foraging area. This leads to the hypothesis that goosanders’ hunting success is lower when cormorants are present in larger numbers, and hence they change their foraging areas. One possible mechanism is that as cormorants are able to hunt socially, larger flocks of cormorants create so much disturbance in the water that fish are scared and/or move (e.g. dive deeper), thus reducing the efficiency of foraging by goosanders. Another probable explanation is kleptoparasitism between piscivorous birds, which has been reported previously between goosanders and grebes (e.g. Cardini and Chiozzi 2015) and may have gone undetected in this study simply due to methodological constraints (duration of bird observation was too short).
The abovementioned relationships between cormorants and goosanders add to our knowledge about sharing of space amongst predators, in this case piscivores. The possibilities of spatial avoidance and indirect or direct competition between bird species using similar food (in this case, fish) should be considered in all investigations of intra- and interspecific ecological relationships. Moreover this study, together with research describing relationships among piscivorous birds, could provide a foundation for future research of economic importance, for example, investigations into the impact of piscivores on angling and fisheries. Both cormorants and goosanders are held responsible for damage to Central European fisheries (e.g. Marquiss and Carss 1994; Carpentier et al. 2003; Mous et al. 2003; Stempniewicz et al. 2003; Wilson et al. 2003; Harris et al. 2008; Östman et al. 2013; Manikowska-Ślepowrońska et al. 2016); however, the impact of the two species is probably different and dependent not only on the season or type of water, but also on the extent to which the two species (and perhaps other piscivores) co-occur.
Further research into these explanations is needed, including determining the exact diet of both species when they are co-located and the spatial movement of birds over longer periods (using radio transmitters and geographic information system analyses). The patterns observed in the Carpathians, and similar patterns observed in adjacent Bohemia (Musil et al. 2015), may not generalise to other areas as conditions in the mountainous areas of Central Europe are specific (limited number and scattered distribution of bodies of water suitable for foraging by goosanders and cormorants) and distinct from those prevailing on large lowland bodies of water (coasts, lakes, large rivers and reservoirs) in Northern and Western Europe, where availability of water and prey probably reduces the competition among piscivorous species dramatically. This phenomenon is expected to be more apparent in future, in response to climate change, when piscivorous birds will winter in northern aquatic areas (Mooij et al. 2005).
References
Amirowicz A, Gwiazda R (2012) How equally sized piscivorous birds and fish sharing common food resources may reduce possible feeding interactions between them? Hydrobiologia 696:95–105
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74:1175–1178
Berger KM, Gese ME (2007) Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol 76:1075–1085
Berthold P (1993) Bird migration: a general survey. Oxford University Press, Oxford
Blázquez M, Sánchez-Zapata JA, Botella F, Carrete M, Eguía S (2009) Spatio-temporal segregation of facultative avian scavengers at ungulate carcasses. Acta Oecol 35:645–650
Bur MT, Stapanian MA, Bernhardt G, Turner MW (2008) Fall diets of red-breasted Merganser (Mergus serrator) and walleye (Sander vitreus) in Sandusky Bay and adjacent waters of Western Lake Erie. Am Midl Nat 159:147–161
Camphuysen CJ (1995) Herring Gull Larus argentatus and Lesser Black-backed Gull L. fuscus feeding at fishing vessels in the breeding season: competitive scavenging versus efficient flying. Ardea 83:365–380
Capizzi D, Luiselli L (1996) Feeding relationships and competitive interactions between phylogenetically unrelated predators (owls and snakes). Acta Oecol 17:265–284
Cardini A, Chiozzi G (2015) Short communications Piracy strikes back on Lake Maggiore (Northern Italy): first report of Common Merganser Mergus merganser kleptoparasitizing Great Crested Grebe Podiceps cristatus. Riv Ital Ornithol Res Ornithol 85:67–72
Carpentier A, Paillisson JM, Marion L (2003) Assessing the interaction between Cormorants and fisheries: the importance of fish community change. In: Cowx IG (ed) Interactions between fish and birds: implications for management. Blackwell Science Ltd, Oxford, pp 187–195
Carss DN, Van Eerden M, Enstipp M, Govedic M, Gwiazda R, Trauttmansdorff J (2012) Cormorant diet, food and energy intake, 4. In: Carss D, Parz-Gollner R, Trauttmansdorff J (eds) Research methods for Cormorants, fishes, and the interactions between them. The INTERCAFE Field Manual, COST Action 635, pp 35–53
Chakarov N, Krüger O (2010) Mesopredator release by an emergent superpredator: a natural experiment of predation in a three level guild. PLoS ONE 5:e15229
Chodkiewicz T, Neubauer G, Chylarecki P, Sikora A, Cenian Z, Ostasiewicz M, Wylęgała P, Ławicki Ł, Smyk B, Betleja J, Gaszewski K, Górski A, Grygoruk G, Kajtoch Ł, Kata K, Krogulec J, Lenkiewicz W, Marczakiewicz P, Nowak D, Pietrasz K, Rohde Z, Rubacha S, Stachyra P, Świętochowski P, Tumiel T, Urban M, Wieloch M, Woźniak B, Zielińska M, Zieliński P (2013) Monitoring populacji ptaków Polski w latach 2012–2013. Biuletyn Monitoringu Przyrody 11:35
Cramp S, Simmons KEL (eds) (1977) The birds of the western Palearctic, vol 1. Oxford University Press, Oxford
Dirksen S, Boudewijn TJ, Noordhuis R, Marteijn ECL (1995) Cormorants Phalacrocorax carbo sinensis in shallow eutrophic freshwater lakes: prey choice and fish consumption in the non-breeding period and effects of large-scale fish removal. Ardea 83:167–184
Gibbons RE, Withers K (2006) Habitat preferences of surface-diving waterbirds and American White Pelicans wintering in Redfish Bay, Texas. Southwest Nat 51:103–107
Gilissen N, Haanstra L, Delany S, Boere G, Hagemeijer W (2002) Numbers and distribution of wintering waterbirds in the Western Palearctic and Southwest Asia in 1997, 1998 and 1999. Results from the International Waterbird Census. Wetlands International Global Series No. 11, Wageningen, The Netherlands
Goldsworthy SD, He X, Tuck GN, Lewis M, Williams R (2001) Trophic interactions between the Patagonian toothfish, its fishery, and seals and seabirds around Macquarie Island. Mar Ecol Prog Ser 218:283–302
Hansen SG (1976) Some aspects of the migration biology of the Goosander Mergus merganser populations in northwestern Europe on basis of the existing ringing data. Danske Fugle 28:164–178
Harris CM, Calladine J, Wernham CW, Park KJ (2008) Impacts of piscivorous birds on salmonid populations and game fisheries in Scotland: a review. Wildl Biol 14:395–411
Kajtoch Ł, Bobrek R (2014) Extension of Goosander Mergus merganser distribution into the Carpathian Mountain range. Wildfowl 64:91–101
Källander H (2008) Flock-fishing in the Great Crested Grebe Podiceps cristatus. Ardea 96:125–128
Keller V (2009) The Goosander Mergus merganser population breeding in the Alps and its connections to the rest of Europe. Wildfowl 2:60–73
Kostrzewa A (1991) Interspecific interference competition in three European raptor species. Ethol Ecol Evol 3:127–143
Manikowska-Ślepowrońska B, Szydzik B, Jakubas D (2016) Determinants of the presence of conflict bird and mammal species at pond fisheries in western Poland. Aquat Ecol 50:87–95
Marquiss M, Carss DN (1994) Avian piscivores: Basis for policy. National Rivers Authority R&D Project 461. National Rivers Authority, Bristol
Marquiss M, Duncan K (1994) Seasonal switching between habitats and changes in abundance of Goosanders Mergus merganser within a Scottish river system. Wildfowl 45:198–208
Mooij WM, Hülsmann S, De Senerpont Domis LN, Nolet BA, Bodelier PLE, Boers PCM, Pires LMD, Gons HJ, Ibelings BW, Noordhuis R (2005) The impact of climate change on lakes in the Netherlands: a review. Aquat Ecol 39:381–400
Morkūnė R (2011) Trophic peculiarities of the Great Cormorant, Grey Heron and Long-tailed Duck on the Baltic Sea Lithuanian coast: a stable isotope approach. Ekologija 57:173–178
Mous PJ, Dekker W, De Leeuw JJ, van Eerden MR, van Densen WLT (2003) Interactions in the utilisation of small fish by piscivorous fish and birds, and the fishery in IJsselmeer. In: Cowx JG (ed) Interaction between fish and birds: implications for management. Blackwell Science Ltd, Oxford, pp 84–118
Musil P, Musilová Z, Fuchs R, Poláková S (2011) Long-term changes in numbers and distribution of wintering waterbirds in the Czech Republic, 1966–2008. Bird Study 58:450–460
Musil P, Ridzoň J, Musilová Z, Slabeyová K (2015) Effect of site variables on the trends in numbers of wintering Cormorants and Goosanders in Central Europe, 1991–2013. Cormor Res Group Bull 8:12
Orben RA, Irons DB, Paredes R, Roby DD, Phillips RA, Shaffer SA (2015) North or south? Niche separation of endemic red-legged kittiwakes and sympatric black-legged kittiwakes during their non-breeding migrations. J Biogeogr 42:401–412
Östman Ö, Boström MK, Bergström U, Andersson J, Lunneryd SG (2013) Estimating competition between wildlife and humans—a case of cormorants and coastal fisheries in the Baltic Sea. PLoS ONE 8:e83763
Paprocki N, Heath JA, Novak SJ (2014) Regional distribution shifts help explain local changes in wintering raptor abundance: implications for interpreting population trends. PLoS ONE 9:e86814
Ross RK (1977) A comparison of the feeding and nesting of the great cormorant (Phalacrocorax carbo L.) and double-crested cormorant (P. auritus Lesson) in Nova Scotia. Proc N S Inst Sci 27:114–133
Rubolini D, Møller AP, Rainio K, Lehikoinen E (2007) Intraspecific consistency and geographical variability in temporal trends of spring migration phenology among European bird species. Clim Res 35:135–146
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Schoener TW (1982) The controversy over interspecific competition. Am Sci 70:586–595
Sergio F, Marchesi L, Pedrini P, Penteriani V (2007) Coexistence of a generalist owl with its intraguild predator: distance-sensitive or habitat-mediated avoidance? Anim Behav 74:1607–1616
Sjöberg K (1985) Foraging activity patterns in the Goosander (Mergus merganser) and the red-breasted merganser (M. serrator) in relation to patterns of activity in their major prey species. Oecologia 67:35–39
Sjöberg K (1988) Food selection, food-seeking patterns and hunting success of captive Goosanders Mergus merganser and Red-breasted Mergansers M. senator in relation to the behaviour of their prey. Ibis 130:79–93
StatSoft Inc. (2001) STATISTICA for Windows. Tulsa
Stempniewicz L, Martyniak A, Borowski W, Goc M (2003) Fish stocks, commercial fishing and Cormorant predation in the Vistula Lagoon, Poland. In: Cowx IG (ed) Interactions between fish and birds: implications for management. Blackwell Science Ltd, Oxford, pp 51–64
Suter W (1995) Are Cormorants Phalacrocorax carbo wintering in Switzerland approaching carrying capacity? An analysis of increase patterns and habitat choice. Ardea 83:255–266
Tannerfeldt M, Elmhagen B, Angerbjörn A (2002) Exclusion by interference competition? The relationship between red and artic foxes. Oecologia 132:213–220
Van Dobben WH (1995) The food of the Cormorant Phalacrocorax carbo sinensis: old and new research compared. Ardea 83:139–142
Van Eerden MR, Voslamber B (1995) Mass fishing by Cormorants Phalacrocorax carbo sinensis at lake Ijsselmeer, The Netherlands: a recent and successful adaptation to a turbid environment. Ardea 83:199–212
Wilk T, Bobrek R, Pępkowska-Król A, Neubauer G, Kosicki JZ (eds) (2016) Ptaki polskich Karpat—stan, zagrożenia, ochrona. OTOP, Marki
Wilson BR, Feltham MJ, Davies JM, Holden T, Cowx JG, Harvey JP, Britton JR (2003) A quantitative assessment of the impact of Goosander, Mergus merganser, on salmonid populations in two upland rivers in English and Wales. In: Cowx IG (ed) Interactions between fish and birds: implications for management. Blackwell Science Ltd, Oxford, pp 119–135
Wood CC, Hand CM (1985) Food-searching behaviour of the common merganser (Mergus merganser) I: functional responses to prey and predator density. Can J Zool 63:1260–1270
Wood KA, Stillman RA (2014) Do birds of a feather flock together? Comparing habitat preferences of piscivorous waterbirds in a lowland river catchment. Hydrobiologia 738:87–95
Žydelis R, Kontautas A (2008) Piscivorous birds as top predators and fishery competitors in the lagoon ecosystem. Hydrobiologia 611:45–54
Acknowledgements
We are grateful for valuable comments of two anonymous reviewers. This study was partly supported by the statutory research of the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, and the Institute of Nature Conservation Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study complies with current Polish, Slovakian, and international laws.
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kajtoch, Ł., Lešo, P., Matysek, M. et al. Do flocks of great cormorants and goosanders avoid spatial overlap in foraging habitat during the non-breeding season?. Aquat Ecol 51, 473–483 (2017). https://doi.org/10.1007/s10452-017-9630-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9630-7