Abstract
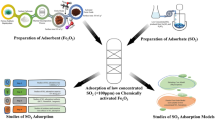
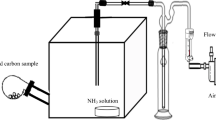
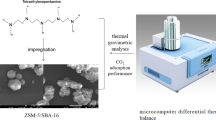
Carbon based nano TiO2-ZnO composite adsorbents were developed and evaluated for simultaneous adsorption of ammonia (NH3) and hydrogen sulphide (H2S). Screening of composites with different ZnO and TiO2 loadings in terms of adsorption capacities identified a composite with 10% ZnO and 5% TiO2 (10ZnO-5TiO2-AC) as the most suitable. Breakthrough experiments with pre-mixed gases containing 50 to 550 mg L− 1 of each NH3 and H2S at 22 to 280 °C showed that increase in NH3 and H2S concentrations led to higher equilibrium adsorption capacities for both gases. Increase of temperature decreased NH3 equilibrium adsorption capacity but for H2S higher values were observed at higher temperatures. The highest equilibrium adsorption capacity of 5.71 mg NH3 g− 1 was obtained with a mixture of 500 ppmv NH3 and 550 ppmv H2S at 22 °C, while for H2S the highest value of 29.64 mg H2S g− 1 was seen with a mixture of 300 ppmv NH3 and 300 ppmv H2S at 280 °C. Multicomponent Langmuir isotherm described the simultaneous adsorption of NH3 and H2S with the high level of accuracy. The negative value of enthalpy of adsorption for NH3 confirmed the exothermic and potentially physical nature of ammonia adsorption, while a positive value for H2S adsorption pointed out to the endothermic and chemisorption nature of this process. Examination of fresh and exposed composite adsorbents by XRD and FTIR confirmed the chemical nature of H2S adsorption.







Similar content being viewed by others
References
Agorku, E.S., Mamo, M.A., Mamba, B.B., Pandey, A.C., Mishra, A.K.: Cobalt-doped ZnS-reduced graphene oxide nanocomposite as an advanced photocatalytic material. J. Porous Mater. 2014. 22(1 22), 47–56 (2014). https://doi.org/10.1007/S10934-014-9871-Y
Alinezhad, E., Haghighi, M., Rahmani, F., Keshizadeh, H., Abdi, M., Naddafi, K.: Technical and economic investigation of chemical scrubber and bio-filtration in removal of H2S and NH3 from wastewater treatment plant. J. Environ. Manage. 241, 32–43 (2019). https://doi.org/10.1016/j.jenvman.2019.04.003
Bhandari, P.N., Kumar, A., Huhnke, R.L.: Simultaneous removal of toluene (model tar), NH3, and H 2S, from biomass-generated producer gas using biochar-based and mixed-metal oxide catalysts. In: Energy and Fuels. pp 1918–1925 (2014)
Chellappa, M., Anjaneyulu, U., Manivasagam, G., Vijayalakshmi, U.: Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int. J. Nanomed. 10, 31–41 (2015). https://doi.org/10.2147/IJN.S79978
Das, J., Nolan, S., Lens, P.N.L.: Simultaneous removal of H2S and NH3 from raw biogas in hollow fibre membrane bioreactors. Environ. Technol. Innov. 28 (2022). https://doi.org/10.1016/j.eti.2022.102777
Elizabeth, M., Cecil, K.K., Talam, E.K.: Hydrogen sulfide and ammonia removal from biogas using water hyacinth-derived carbon nanomaterials. Afr. J. Environ. Sci. Technol. 11(7), 375–383 (2017). https://doi.org/10.5897/ajest2016.2246
Foo, K.Y., Hameed, B.H.: Decontamination of textile wastewater via TiO2 /activated carbon composite materials. Adv. Colloid Interface Sci. 159(2), 130–143 (2010). https://doi.org/10.1016/J.CIS.2010.06.002
Gandu, B., Palanivel, S., Juntupally, S., Arelli, V., Begum, S., Anupoju, G.R.: Removal of NH3 and H2S from odor causing tannery emissions using biological filters: Impact of operational strategy on the performance of a pilot-scale bio-filter. J. Environ. Sci. Health Part. A. 56(6), 625–634 (2021). https://doi.org/10.1080/10934529.2021.1903283
Hernández, J., Dorado, A.D., Lafuente, J., Gamisans, X., Prado, Ó.J., Gabriel, D.: Characterization and evaluation of poplar and pine wood in twin biotrickling filters treating a mixture of NH3, H2S, butyric acid, and ethylmercaptan. Environ. Progress Sustainable Energy. 36(1), 171–179 (2017). https://doi.org/10.1002/ep.12491
Huan, C., Fang, J., Tong, X., Zeng, Y., Liu, Y., Jiang, X., Ji, G., Xu, L., Lyu, Q., Yan, Z.: Simultaneous elimination of H2S and NH3 in a biotrickling filter packed with polyhedral spheres and best efficiency in compost deodorization. J. Clean. Prod. 284, 124708 (2021). https://doi.org/10.1016/j.jclepro.2020.124708
Jamil, T.S., Ghaly, M.Y., Fathy, N.A., Abd El-Halim, T.A., Österlund, L.: Enhancement of TiO2 behavior on photocatalytic oxidation of MO dye using TiO2/AC under visible irradiation and sunlight radiation. Sep. Purif. Technol. 98, 270–279 (2012). https://doi.org/10.1016/J.SEPPUR.2012.06.018
Jamshidi, M., Ghaedi, M., Dashtian, K., Ghaedi, A.M., Hajati, S., Goudarzi, A., Alipanahpour, E.: Highly efficient simultaneous ultrasonic assisted adsorption of brilliant green and eosin B onto ZnS nanoparticles loaded activated carbon: Artificial neural network modeling and central composite design optimization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 153, 257–267 (2016). https://doi.org/10.1016/J.SAA.2015.08.024
Jiang, Q., Li, T., He, Y., Wu, Y., Zhang, J., Jiang, M.: Simultaneous removal of hydrogen sulfide and ammonia in the gas phase: A review. Environ. Chem. Lett. 20, 1403–1419 (2022)
Jung, S.Y., Lee, S.J., Park, J.J., Lee, S.C., Jun, H.K., Lee, T.J., Ryu, C.K., Kim, J.C.: The simultaneous removal of hydrogen sulfide and ammonia over zinc-based dry sorbent supported on alumina. Sep. Purif. Technol. 63(2), 297–302 (2008). https://doi.org/10.1016/J.SEPPUR.2008.05.013
Lim, E.J., Jung, S.Y., Lee, S.C., Kim, J.C.: Enhancing the effect of CoAl2O4 on the simultaneous removal of H2S and NH3 on Co- and Mo- based catal-sorbents in IGCC. Sep. Purif. Technol. 177, 94–100 (2017). https://doi.org/10.1016/j.seppur.2016.11.055
Lu, X., Jiang, J., Sun, K., Cui, D.: Characterization and photocatalytic activity of Zn2+–TiO2/AC composite photocatalyst. Appl. Surf. Sci. 258(5), 1656–1661 (2011). https://doi.org/10.1016/J.APSUSC.2011.09.042
Makarova, O., Rajh, T., Thurnauer, M.C., Martin, A., Kemme, P.A., Cropek, D.: Surface modification of TiO2 nanoparticles for photochemical reduction of nitrobenzene. Environ. Sci. Technol. 34(22), 4797–4803 (2000). https://doi.org/10.1021/es001109+
Neelgund, G.M., Oki, A.: Photothermal effect: An important aspect for the enhancement of photocatalytic activity under illumination by NIR radiation. Mater. Chem. Front. 2(1), 64–75 (2017). https://doi.org/10.1039/C7QM00337D
Ouzzine, M., Romero-Anaya, A.J., Lillo-Ródenas, M.A., Linares-Solano, A.: Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon. 67, 104–118 (2014). https://doi.org/10.1016/J.CARBON.2013.09.069
Park, J.J., Park, C.G., Jung, S.Y., Lee, S.C., Ragupathy, D., Kim, J.C.: A study on Zn-based catal-sorbents for the simultaneous removal of hydrogen sulfide and ammonia at high temperature. In: Research on Chemical Intermediates. pp 1193–1202 (2011)
Piña-Pérez, Y., Aguilar-Martínez, O., Acevedo-Peña, P., Santolalla-Vargas, C.E., Oros-Ruíz, S., Galindo-Hernández, F., Gómez, R., Tzompantzi, F.: Novel ZnS-ZnO composite synthesized by the solvothermal method through the partial sulfidation of ZnO for H2 production without sacrificial agent. Appl. Catal. B. 230, 125–134 (2018). https://doi.org/10.1016/J.APCATB.2018.02.047
Rezaei, E., Azar, R., Nemati, M., Predicala, B.: Gas phase adsorption of ammonia using nano TiO2-activated carbon composites – effect of TiO2 loading and composite characterization. J. Environ. Chem. Eng. 5(6), 5902–5911 (2017a). https://doi.org/10.1016/J.JECE.2017.11.010
Rezaei, E., Schlageter, B., Nemati, M., Predicala, B.: Evaluation of metal oxide nanoparticles for adsorption of gas phase ammonia. J. Environ. Chem. Eng. 5(1), 422–431 (2017b). https://doi.org/10.1016/J.JECE.2016.12.026
Rosso, I., Galletti, C., Bizzi, M., Saracco, G., Specchia, V.: Zinc oxide sorbents for the removal of hydrogen sulfide from syngas. Ind. Eng. Chem. Res. 42(8), 1688–1697 (2003). https://doi.org/10.1021/ie0208467
Song, H.S., Park, M.G., Kwon, S.J., Yi, K.B., Croiset, E., Chen, Z., Nam, S.C.: Hydrogen sulfide adsorption on nano-sized zinc oxide/reduced graphite oxide composite at ambient condition. Appl. Surf. Sci. 276, 646–652 (2013). https://doi.org/10.1016/J.APSUSC.2013.03.147
Valdes Labrada, G.M., Kumar, S., Azar, R., Predicala, B., Nemati, M.: Simultaneous capture of NH3 and H2S using TiO < inf > 2 and ZnO nanoparticles - laboratory evaluation and application in a livestock facility. J. Environ. Chem. Eng. 8(1) (2020). https://doi.org/10.1016/j.jece.2019.103615
Xue, G., Liu, H., Chen, Q., Hills, C., Tyrer, M., Innocent, F.: Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard. Mater. 186(1), 765–772 (2011). https://doi.org/10.1016/J.JHAZMAT.2010.11.063
Zhang, Z., Xu, Y., Ma, X., Li, F., Liu, D., Chen, Z., Zhang, F., Dionysiou, D.D.: Microwave degradation of methyl orange dye in aqueous solution in the presence of nano-TiO2-supported activated carbon (supported-TiO2/AC/MW). J. Hazard. Mater. 209–210, 271–277 (2012). https://doi.org/10.1016/J.JHAZMAT.2012.01.021
Zheng, T., Li, L., Chai, F., Wang, Y.: Factors impacting the performance and microbial populations of three biofilters for co-treatment of H2S and NH3 in a domestic waste landfill site. Process Saf. Environ. Prot. 149, 410–421 (2021). https://doi.org/10.1016/j.psep.2020.11.009
Acknowledgements
This work was made possible by an Agriculture Development Fund grant (ADF 20140246) from the Ministry of Agriculture, Government of Saskatchewan. Technical assistance of R. Blondin, and R. Prokopishyn from the Department of Chemical and Biological Engineering, University of Saskatchewan is gratefully acknowledged.
Funding
Agriculture Development Fund grant (ADF 20140246), Ministry of Agriculture, Government of Saskatchewan, Canada.
Author information
Authors and Affiliations
Contributions
Guadalupe Montserrat Valdes Labrada: Methodology, Investigation, Data interpretation and validation. Ruth Azar: Methodology, Investigation, Data interpretation and validation.Bernardo Predicala: Conceptualization, Methodology, Co-supervision. Mehdi Nemati: Conceptualization, Methodology, Co-supervision, Research management, Writing-first draft, revisions, submission, and correspondence. All authors contributed in writing the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labrada, G.M.V., Azar, R., Predicala, B. et al. Mitigation of hazardous ammonia and hydrogen sulphide emissions using carbon based nanometal oxides adsorbents. Adsorption (2024). https://doi.org/10.1007/s10450-024-00474-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00474-7