Abstract
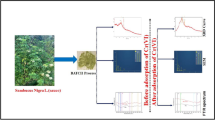
In this research, a one-step xanthation process was used to synthesize biosorbents from persimmon leaves (Diospyros kaki). The resulting biosorbents, referred to as MPM, exhibited high sorption capacities for Hexavalent chromium and Cd(II) at different pH values. Specifically, at pH 3, the Langmuir maximum adsorption capacity for Cr(VI)-MPM was determined to be 710 mg/g, while at pH 7, it was 622 mg/g for Cd(II)-MPM systems. The adsorption kinetics of both the metal ions followed the pseudo-2nd-order model, with R2 values close to 1 (0.99). Through FT-IR and XPS studies, it was determined that ion exchange, surface complexation, and chelation were the primary mechanisms responsible for removing the analytes from water using MPM as sorbent. The presence of the -CS2-Na group in MPM played a crucial role in these removal mechanisms. Additionally, MPM displayed notable antibacterial efficacy against S. aureus and E. coli. Considering its rapid kinetics, wide pH range applicability, impressive sorption capacity, recyclability, and effective antibacterial properties, MPM proves to be a highly suitable adsorbent for removing Hexavalent chromium and Cd(II) from industrial wastewater.






Similar content being viewed by others
Data availability
Data will be provided upon reasonable request.
References
Ahmadi, H., Hafiz, S.S., Sharifi, H., Rene, N.N., Habibi, S.S., Hussain, S.: Low cost biosorbent (Melon Peel) for effective removal of Cu (II), Cd (II), and Pb (II) ions from aqueous solution. Case Studies Chem. Environ. Eng. 6, 100242 (2022). https://doi.org/10.1016/j.cscee.2022.100242
Ahsan, M.A., Katla, S.K., Islam, M.T., Hernandez-Viezcas, J.A., Martinez, L.M., Díaz-Moreno, C.A., Noveron, J.C.: Adsorptive removal of methylene blue, tetracycline and Cr (VI) from water using sulfonated tea waste. Environ. Technol. Innov. 11, 23–40 (2018). https://doi.org/10.1016/j.eti.2018.04.003
Ampiaw, R.E., Lee, W.: Persimmon tannins as biosorbents for precious and heavy metal adsorption in wastewater: a review. Int. J. Environ. Sci. Technol. 17, 3835–3846 (2020). https://doi.org/10.1007/s13762-020-02748-3
Anakhu, E.A., Ameh, V.I., Modekwe, H.U., Ayeleru, O.O., Ramatsa, I.M.: Remediation of cadmium and chromium using modified Vitex doniana waste plant Seed’s biochar in quarry site surface water. Environ. Funct. Mater. (2024). https://doi.org/10.1016/j.efmat.2024.02.002
Arslan, D.Ş., Ertap, H., Şenol, Z.M., El Messaoudi, N., Mehmeti, V.: Preparation of polyacrylamide titanium dioxide hybrid nanocomposite by direct polymerization and its applicability in removing crystal violet from aqueous solution. J. Polym Environ. 1–15 (2023). https://doi.org/10.1007/s10924-023-03004-8
Arim, A.L., Quina, M.J., Gando-Ferreira, L.M.: Uptake of trivalent chromium from aqueous solutions by xanthate pine bark: characterization, batch and column studies. Process Saf. Environ. Prot. 121, 374–386 (2019). https://doi.org/10.1016/j.psep.2018.11.001
Biesinger, M.C., Brown, C., Mycroft, J.R., Davidson, R.D., McIntyre, N.S.: X-ray photoelectron spectroscopy studies of chromium compounds. Surface Interf. Anal.: Int. J. Devoted Dev. Appl. Tech. Anal. Surfaces, Interf. Thin Films 36(12), 1550–1563 (2004). https://doi.org/10.1002/sia.1983
Bilal, M., Ihsanullah, I., Younas, M., Shah, M.U.H.: Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: a critical review. Sep. Purif. Technol. 278, 119510 (2021). https://doi.org/10.1016/j.seppur.2021.119510
Chao, G., Liu, Y., Tan, X., Wang, S., Zeng, G., Zheng, B., Wei, L.: Effect of porous zinc–biochar nanocomposites on Cr (VI) adsorption from aqueous solution [J]. RSC Adv. 5, 35107–35115 (2015)
Chand, P., Bafana, A., Pakade, Y.B.: Xanthate modified apple pomace as an adsorbent for removal of Cd (II), Ni (II) and Pb (II), and its application to real industrial wastewater. Int. Biodeterior. Biodegradation 97, 60–66 (2015). https://doi.org/10.1016/j.ibiod.2014.10.015
Chauhan, D., Afreen, S., Mishra, S., Sankararamakrishnan, N.: Synthesis, characterization and application of zinc augmented aminated PAN nanofibers towards decontamination of chemical and biological contaminants. J. Ind. Eng. Chem. 55, 50–64 (2017). https://doi.org/10.1016/j.jiec.2017.06.027
Chen, D., Wang, X., Wang, X., Feng, K., Su, J., Dong, J.: The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 714, 136550 (2020). https://doi.org/10.1016/j.scitotenv.2020.136550
Córdova, B.M., Venâncio, T., Olivera, M., Huamani-Palomino, R.G., Valderrama, A.C.: Xanthation of alginate for heavy metal ions removal. Characterization of xanthate-modified alginates and its metal derivatives. Int. J. Biol. Macromol. 169, 130–142 (2021). https://doi.org/10.1016/j.ijbiomac.2020.12.022
Dong, X., Ma, L.Q., Li, Y.: Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 190(1–3), 909–915 (2011). https://doi.org/10.1016/j.jhazmat.2011.04.008
El Messaoudi, N., Cigeroglu, Z., Şenol, Z.M., Bouich, A., Kazan-Kaya, E.S., Noureen, L., Américo-Pinheiro, J.H.P.: Chapter Fourteen Green synthesis of nanoparticles for remediation organic pollutants in wastewater by adsorption. In: Kumar, A., Bilal, M., Ferreira, L.F.R. (eds.) In Advances in Chemical Pollution, Environmental Management and Protection, vol. 10, pp. 305–345. Elsevier (2023). https://doi.org/10.1016/bs.apmp.2023.06.016
El Messaoudi, N., Cigeroglu, Z., Şenol, Z.M., Elhajam, M., Noureen, L.: A comparative review of the adsorption and photocatalytic degradation of tetracycline in aquatic environment by g-C3N4-based materials. J. Water Process Eng. 55, 104150 (2023). https://doi.org/10.1016/j.jwpe.2023.104150
El Messaoudi, N., Ciğeroğlu, Z., Şenol, Z.M., Kazan-Kaya, E.S., Fernine, Y., Gubernat, S., Lopicic, Z.: Green synthesis of CuFe2O4 nanoparticles from bioresource extracts and their applications in different areas: a review. Biomass Convers. Biorefinery 1–22 (2024). https://doi.org/10.1007/s13399-023-05264-9
El Mouden, A., El Messaoudi, N., El Guerraf, A., Bouich, A., Mehmeti, V., Lacherai, A., ... Américo-Pinheiro, J.H.P.: Removal of cadmium and lead ions from aqueous solutions by novel dolomite-quartz@ Fe3O4 nanocomposite fabricated as nanoadsorbent. Environ. Res. 225, 115606 (2023). https://doi.org/10.1016/j.envres.2023.115606
El Mouden, A., El Guerraf, A., El Messaoudi, N., Haounati, R., Ait El Fakir, A., Lacherai, A.: Date stone functionalized with 3-aminopropyltriethoxysilane as a potential biosorbent for heavy metal ions removal from aqueous solution. Chem. Africa 5(3), 745–759 (2022). https://doi.org/10.1007/s42250-022-00350-3
El Khomri, M., El Messaoudi, N., Dbik, A., Bentahar, S., Lacherai, A., Chegini, Z.G., Bouich, A.: Removal of Congo red from aqueous solution in single and binary mixture systems using Argan nutshell wood. Pigm. Resin Technol. 51(5), 477–488 (2021). https://doi.org/10.1108/PRT-04-2021-0045
Fan, R., Yi, Q., Xie, Y., Xie, F., Zhang, Q., Luo, Z. (2016). Enhanced adsorption and recovery of Pb (II) from aqueous solution by alkali‐treated persimmon fallen leaves. J. Appl. Polym. Sci. 133(28). https://doi.org/10.1002/app.43656
Feng, Y., Du, Y., Chen, Z., Du, M., Yang, K., Lv, X., Li, Z.: Synthesis of Fe 3 O 4 nanoparticles with tunable sizes for the removal of Cr (VI) from aqueous solution. J. Coat. Technol. Res. 15, 1145–1155 (2018). https://doi.org/10.1007/s11998-018-0052-9
Garg, R., Garg, R., Sillanpää, M., Alimuddin, Khan, M.A., Mubarak, N.M., Tan, Y.H.: Rapid adsorptive removal of chromium from wastewater using walnut-derived biosorbents. Sci. Rep. 13(1), 6859 (2023). https://doi.org/10.1038/s41598-023-33843-3
Gong, W.X., Qu, J.H., Liu, R.P., Lan, H.C.: Effect of aluminum fluoride complexation on fluoride removal by coagulation. Colloids Surf., A 395, 88–93 (2012). https://doi.org/10.1016/j.colsurfa.2011.12.010
Gupta, A., Sharma, V., Sharma, K., Kumar, V., Choudhary, S., Mankotia, P., ... Mishra, P. K.: A review of adsorbents for heavy metal decontamination: growing approach to wastewater treatment. Materials 14(16), 4702 (2021). https://doi.org/10.3390/ma14164702
Gupta, S., Garg, D., Kumar, A.: Cadmium biosorption using Aloe. barbadensis Miller leaves waste powder treated with sodium bicarbonate. Clean. Waste Syst. 3, 100032 (2022). https://doi.org/10.1016/j.clwas.2022.100032
Irshad, Z., Bibi, I., Ghafoor, A., Majid, F., Kamal, S., Ezzine, S., Iqbal, M.: Ni doped SrFe12O19 nanoparticles synthesized via micro-emulsion route and photocatalytic activity evaluation for the degradation of crystal violet under visible light irradiation. Results Phys. 42, 106006 (2022). https://doi.org/10.1016/j.rinp.2022.106006
Joseph, L., Jun, B.M., Flora, J.R., Park, C.M., Yoon, Y.: Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229, 142–159 (2019). https://doi.org/10.1016/j.chemosphere.2019.04.198
Kim, H.S., Jeong, S.S., Lee, J.G., Yoon, J.H., Lee, S.P., Kim, K.R., Yang, J.E.: Biologically produced sulfur as a novel adsorbent to remove Cd2+ from aqueous solutions. J. Hazard. Mater. 419, 126470 (2021). https://doi.org/10.1016/j.jhazmat.2021.126470
Lee, S.Y., Choi, H.J.: Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manage. 209, 382–392 (2018). https://doi.org/10.1016/j.jenvman.2017.12.080
Liang, S., Guo, X., Feng, N., Tian, Q.: Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions. J. Hazard. Mater. 170(1), 425–429 (2009). https://doi.org/10.1016/j.jhazmat.2009.04.078
Lippitz, A., Hübert, T.: XPS investigations of chromium nitride thin films. Surf. Coat. Technol. 200(1–4), 250–253 (2005). https://doi.org/10.1016/j.surfcoat.2005.02.091
Liu, C., Virginie, M., Griboval-Constant, A., Khodakov, A.Y.: Potassium promotion effects in carbon nanotube supported molybdenum sulfide catalysts for carbon monoxide hydrogenation. Catal. Today 261, 137–145 (2016). https://doi.org/10.1016/j.cattod.2015.07.003
Liu, F., Hua, S., Wang, C., Hu, B.: Insight into the performance and mechanism of persimmon tannin functionalized waste paper for U (VI) and Cr (VI) removal. Chemosphere 287, 132199 (2022). https://doi.org/10.1016/j.chemosphere.2021.132199
Li, Q., Zhang, Y., Liao, Y., Huang, J., Dang, Z., Guo, C.: Removal of hexavalent chromium using biogenic mackinawite (FeS)-deposited kaolinite. J. Colloid Interface Sci. 572, 236–245 (2020). https://doi.org/10.1016/j.jcis.2020.03.077
Liu, Y., Shan, H., Pang, Y., Zhan, H., Zeng, C.: Iron modified chitosan/coconut shell activated carbon composite beads for Cr (VI) removal from aqueous solution. Int. J. Biol. Macromol. 224, 156–169 (2023). https://doi.org/10.1016/j.ijbiomac.2022.10.112
Lv, D., Zhou, X., Zhou, J., Liu, Y., Li, Y., Yang, K., Xu, X.: Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium (II) removal from aqueous solutions. Appl. Surf. Sci. 442, 114–123 (2018). https://doi.org/10.1016/j.apsusc.2018.02.085
Lu, X., Wu, J., Guo, Y.: Removal of Cd (II) from aqueous solution by sulfur-functionalized walnut shell: adsorption performance and micro-structural morphology. Desalination Water Treat 169, 322–332 (2019). https://doi.org/10.5004/dwt.2019.24742
Mahmoud, M.E., El-Sharkawy, R.M., Ibrahim, G.A.: Promoted adsorptive removal of chromium (vi) ions from water by a green-synthesized hybrid magnetic nanocomposite (NFe 3O4 Starch-Glu-NFe3O4 ED). RSC Adv. 11(24), 14829–14843 (2021). https://doi.org/10.1039/D1RA00961C
Moreno-López, A.Y., González-López, M.E., Manríquez-González, R., González-Cruz, R., Pérez-Fonseca, A.A., Gómez, C., Robledo-Ortíz, J.R.: Evaluation of the Cr (VI) adsorption performance of xanthate polysaccharides supported onto agave fiber-LDPE foamed composites. Water Air Soil Pollut. 230, 1–21 (2019). https://doi.org/10.1007/s11270-019-4181-2
Othmani, A., Magdouli, S., Kumar, P.S., Kapoor, A., Chellam, P.V., Gökkuş, Ö.: Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: A review. Environ. Res. 204, 111916 (2022). https://doi.org/10.1016/j.envres.2021.111916
Paralikar, P., Rai, M.: Bio-inspired synthesis of sulphur nanoparticles using leaf extract of four medicinal plants with special reference to their antibacterial activity. IET Nanobiotechnol. 12(1), 25–31 (2018). https://doi.org/10.1049/iet-nbt.2017.0079
Pillai, S.S., Deepa, B., Abraham, E., Girija, N., Geetha, P., Jacob, L., Koshy, M.: Biosorption of Cd (II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 98, 352–360 (2013). https://doi.org/10.1016/j.ecoenv.2013.09.003
Purrostam, S., Rahimi-Ahar, Z., Babapoor, A., Nematollahzadeh, A., Salahshoori, I., Seyfaee, A.: Melamine functionalized mesoporous silica SBA-15 for separation of chromium (VI) from wastewater. Mater. Chem. Phys. 307, 128240 (2023). https://doi.org/10.1016/j.matchemphys.2023.128240
Qu, J., Meng, X., Zhang, Y., Meng, Q., Tao, Y., Hu, Q., Shoemaker, C.A.: A combined system of microwave-functionalized rice husk and poly-aluminium chloride for trace cadmium-contaminated source water purification: exploration of removal efficiency and mechanism. J. Hazard. Mater. 379, 120804 (2019). https://doi.org/10.1016/j.jhazmat.2019.120804
Raj, R., Dalei, K., Chakraborty, J., Das, S.: Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 462, 166–175 (2016). https://doi.org/10.1016/j.jcis.2015.10.004
Sankararamakrishnan, N., Dixit, A., Iyengar, L., Sanghi, R.: Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Biores. Technol. 97(18), 2377–2382 (2006). https://doi.org/10.1016/j.biortech.2005.10.024
Sankararamakrishnan, N., Shankhwar, A., Chauhan, D.: Mechanistic insights on immobilization and decontamination of hexavalent chromium onto nano MgS/FeS doped cellulose nanofibres. Chemosphere 228, 390–397 (2019). https://doi.org/10.1016/j.chemosphere.2019.04.166
Şenol, Z.M., Elma, E., El Messaoudi, N., Mehmeti, V.: Performance of cross-linked chitosan-zeolite composite adsorbent for removal of Pb2+ ions from aqueous solutions: experimental and Monte Carlo simulations studies. J. Mol. Liq. 391, 123310 (2023). https://doi.org/10.1016/j.molliq.2023.123310
Şenol, Z.M., Messaoudi, N.E., Fernine, Y., Keskin, Z.S.: Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): experimental and DFT modeling studies. Biomass Convers Biorefinery 1–14 (2023). https://doi.org/10.1007/s13399-023-03781-1
Shi, X., Qiao, Y., An, X., Tian, Y., Zhou, H.: High-capacity adsorption of Cr (VI) by lignin-based composite: characterization, performance and mechanism. Int. J. Biol. Macromol. 159, 839–849 (2020). https://doi.org/10.1016/j.ijbiomac.2020.05.130
Szczurek, A., Amaral-Labat, G., Fierro, V., Pizzi, A., Celzard, A.: Chemical activation of tannin-based hydrogels by soaking in KOH and NaOH solutions. Microporous Mesoporous Mater. 196, 8–17 (2014). https://doi.org/10.1016/j.micromeso.2014.04.051
Thabede, P.M., Shooto, N.D., Xaba, T., Naidoo, E.B.: Adsorption studies of toxic cadmium (II) and chromium (VI) ions from aqueous solution by activated black cumin (Nigella sativa) seeds. J. Environ. Chem. Eng. 8(4), 104045 (2020). https://doi.org/10.1016/j.jece.2020.104045
Tor, A.: Removal of fluoride from water using anion-exchange membrane under Donnan dialysis condition. J. Hazard. Mater. 141(3), 814–818 (2007). https://doi.org/10.1016/j.jhazmat.2006.07.043
Viswanathan, N., Meenakshi, S.: Role of metal ion incorporation in ion exchange resin on the selectivity of fluoride. J. Hazard. Mater. 162(2–3), 920–930 (2009). https://doi.org/10.1016/j.jhazmat.2008.05.118
Wang, C., Bi, L., Liu, J., Huang, B., Wang, F., Zhang, Y., ... Song, M.: Microalgae-derived carbon quantum dots mediated formation of metal sulfide nano-adsorbents with exceptional cadmium removal performance. J. Colloid Interface Sci. 629, 994–1002 (2023). https://doi.org/10.1016/j.jcis.2022.08.188
Wang, J., Wang, X., Zhao, G., Song, G., Chen, D., Chen, H., Wang, X.: Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 334, 569–578 (2018). https://doi.org/10.1016/j.cej.2017.10.068
Wang, N., Xu, X., Li, H., Zhai, J., Yuan, L., Zhang, K., Yu, H.: Preparation and application of a xanthate-modified thiourea chitosan sponge for the removal of Pb (II) from aqueous solutions. Ind. Eng. Chem. Res. 55(17), 4960–4968 (2016). https://doi.org/10.1021/acs.iecr.6b00694
Wang, Q., Zheng, C., Cui, W., He, F., Zhang, J., Zhang, T.C., He, C.: Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem. Eng. J. 391, 123581 (2020). https://doi.org/10.1016/j.cej.2019.123581
Wu, Z., Zhou, W., Deng, W., Xu, C., Cai, Y., Wang, X.: Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl. Mater. Interfaces. 12(18), 20307–20320 (2020). https://doi.org/10.1021/acsami.0c05430
Xu, Q., Wang, Y., Jin, L., Wang, Y., Qin, M.: Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 339, 91–99 (2017). https://doi.org/10.1016/j.jhazmat.2017.06.005
Yang, K., Wang, G., Chen, X., Wang, X., Liu, F.: Treatment of wastewater containing Cu2+ using a novel macromolecular heavy metal chelating flocculant xanthated chitosan. Colloids Surf., A 558, 384–391 (2018). https://doi.org/10.1016/j.colsurfa.2018.06.082
Yang, K., Wang, G., Liu, F., Wang, X., Chen, X.: Removal of multiple heavy metal ions using a macromolecule chelating flocculant xanthated chitosan. Water Sci. Technol. 79(12), 2289–2297 (2019). https://doi.org/10.2166/wst.2019.230
Yi, Q., Fan, R., Xie, F., Min, H., Zhang, Q., Luo, Z.: Selective recovery of Au (III) and Pd (II) from waste PCBs using ethylenediamine modified persimmon tannin adsorbent. Procedia Environ. Sci. 31, 185–194 (2016). https://doi.org/10.1016/j.proenv.2016.02.025
Yu, S.W., Choi, H.J.: Application of hybrid bead, persimmon leaf and chitosan for the treatment of aqueous solution contaminated with toxic heavy metal ions. Water Sci. Technol. 78(4), 837–847 (2018). https://doi.org/10.2166/wst.2018.354
Zhang, S., Dang, J., Lin, J., Liu, M., Zhang, M., Chen, S.: Selective enrichment and separation of Ag (I) from electronic waste leachate by chemically modified persimmon tannin. J. Environ. Chem. Eng. 9(1), 104994 (2021). https://doi.org/10.1016/j.jece.2020.104994
Zheng, L., Peng, D., Meng, P.: Corncob-supported aluminium-manganese binary oxide composite enhanced removal of cadmium ions. Colloids Surf., A 561, 109–119 (2019). https://doi.org/10.1016/j.colsurfa.2018.10.075
Zhu, G., Liu, J., Yin, J., Li, Z., Ren, B., Sun, Y., Liu, Y.: Functionalized polyacrylamide by xanthate for Cr (VI) removal from aqueous solution. Chem. Eng. J. 288, 390–398 (2016). https://doi.org/10.1016/j.cej.2015.12.043
Acknowledgements
Department of Chemistry, Banasthali Vidyapith, Rajasthan, and Thematic Unit of Excellence on Soft Nanofabrication, Advance Centre for Material Science, and Advanced Imaging Centre at IIT Kanpur are acknowledged for various characterization studies.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Nalini Sankararamakrishnan: Conceptualization, Methodology, Visualization, Investigation, Supervision, Writing—original draft, review & editing. Neha Singh: Data curation, Investigation, Writing – original draft, review & editing. Sanjana Tewari: Data curation, Investigation, Validation Writing—review & editing. Jaya Dwivedi: Data Validation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable, because this article does not contain any studies with human or animal subjects.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tewari, S., Singh, N., Dwivedi, J. et al. Synthesis, characterization and application of xanthated Diospyros kaki (persimmon) leaves for the treatment of chemical and biological contaminants in aqueous solutions. Adsorption (2024). https://doi.org/10.1007/s10450-024-00470-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00470-x