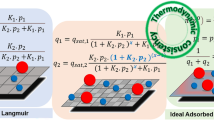
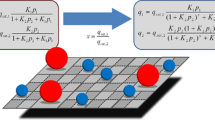
Abstract
Simulation and design of adsorptive separation units demand accurate estimation of thermodynamic properties. Isosteric heat of adsorption as calculated from generalized Langmuir (gL) isotherm coupled with Clausius–Clapeyron expression for pure component and mixed-gas adsorption equilibria is presented in this work. The estimated isosteric heat of adsorption as functions of surface loading and composition is validated against the experimental data for various adsorption systems. Furthermore, the gL results are compared against classical Langmuir (cL) and Toth isotherm for pure components and with Ideal Adsorbed Solution Theory (IAST) for mixed-gas adsorption equilibria. The comparison highlights that gL outperforms cL and Toth for pure component adsorption and IAST for mixed-gas adsorption, and gL reliably captures the loading dependence and the composition dependence for isosteric heat of adsorption.










Similar content being viewed by others
References
Yang, R.T.: Gas Separation by Adsorption Processes. Butterworth-Heinemann, Oxford (2013)
Sundaram, N., Yang, R.T.: Isosteric heats of adsorption from gas mixtures. J. Colloid Interface Sci. 198(2), 378–388 (1998). https://doi.org/10.1006/jcis.1997.5300
Sees, M.D., Kirkes, T., Chen, C.-C.: A simple and practical process modeling methodology for pressure swing adsorption. Comput. Chem. Eng. 147, 107235 (2021). https://doi.org/10.1016/j.compchemeng.2021.107235
Sircar, S.: Role of adsorbent heterogeneity on mixed gas adsorption. Ind. Eng. Chem. Res. 30(5), 1032–1039 (1991). https://doi.org/10.1021/ie00053a027
Shen, D., Bülow, M., Siperstein, F., Engelhard, M., Myers, A.L.: Comparison of experimental techniques for measuring isosteric heat of adsorption. Adsorption 6(4), 275–286 (2000)
Dunne, J.A., Mariwala, R., Rao, M., Sircar, S., Gorte, R.J., Myers, A.L.: Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on Silicalite. Langmuir 12(24), 5888–5895 (1996). https://doi.org/10.1021/la960495z
Sircar, S., Mohr, R., Ristic, C., Rao, M.B.: Isosteric heat of adsorption: theory and experiment. J. Phys. Chem. B 103(31), 6539–6546 (1999). https://doi.org/10.1021/jp9903817
Dunne, J.A., Rao, M., Sircar, S., Gorte, R.J., Myers, A.L.: Calorimetric heats of adsorption and adsorption isotherms. 2. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on NaX, H-ZSM-5, and Na-ZSM-5 Zeolites. Langmuir 12(24), 5896–5904 (1996). https://doi.org/10.1021/la960496r
Siperstein, F., Gorte, R.J., Myers, A.L.: Measurement of excess functions of binary gas mixtures adsorbed in zeolites by adsorption calorimetry. Adsorption 5(2), 169–176 (1999). https://doi.org/10.1023/a:1008973409819
Siperstein, F., Gorte, R.J., Myers, A.L.: A new calorimeter for simultaneous measurements of loading and heats of adsorption from gaseous mixtures. Langmuir 15(4), 1570–1576 (1999). https://doi.org/10.1021/la980946a
Sircar, S., Golden, T.C.: 110th Anniversary: comments on heterogeneity of practical adsorbents. Ind. Eng. Chem. Res. 58(25), 10984–11002 (2019). https://doi.org/10.1021/acs.iecr.9b01025
Sircar, S.: Heat of adsorption on heterogeneous adsorbents. Appl. Surf. Sci. 252(3), 647–653 (2005). https://doi.org/10.1016/j.apsusc.2005.02.082
Tun, H., Chen, C.-C.: Isosteric heat of adsorption from thermodynamic Langmuir isotherm. Adsorption 27(6), 979–989 (2021). https://doi.org/10.1007/s10450-020-00296-3
Son, K.N., Cmarik, G.E., Knox, J.C., Weibel, J.A., Garimella, S.V.: Measurement and prediction of the heat of adsorption and equilibrium concentration of CO2 on zeolite 13X. J. Chem. Eng. Data 63(5), 1663–1674 (2018). https://doi.org/10.1021/acs.jced.8b00019
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918). https://doi.org/10.1021/ja02242a004
Mathias, P.M., Kumar, R., Moyer, J.D., et al.: Correlation of multicomponent gas adsorption by the dual-site Langmuir model. Application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 35(7), 2477–2483 (1996)
Toth, J.: State equation of the solid-gas interface layers. Acta Chim Hung. 69, 311–328 (1971)
Chang, C.-K., Tun, H., Chen, C.-C.: An activity-based formulation for Langmuir adsorption isotherm. Adsorption 26(3), 375–386 (2020). https://doi.org/10.1007/s10450-019-00185-4
Kaur, H., Tun, H., Sees, M., Chen, C.-C.: Local composition activity coefficient model for mixed-gas adsorption equilibria. Adsorption 25(5), 951–964 (2019). https://doi.org/10.1007/s10450-019-00127-0
Sircar, S.: Excess properties and thermodynamics of multicomponent gas adsorption. J. Chem. Soc. Faraday Trans. 81(7), 1527–1540 (1985). https://doi.org/10.1039/f19858101527
He, Y., Yun, J.H., Seaton, N.A.: Adsorption equilibrium of binary methane/ethane mixtures in BPL activated carbon: isotherms and calorimetric heats of adsorption. Langmuir 20(16), 6668–6678 (2004). https://doi.org/10.1021/la036430v
Bulow, M., Lorenz, P.: Isosteric adsorption equilibria for binary krypton-xenon mixtures on CaA type zeolite. Ind. Eng. Chem. Res. 61, 119–128 (1987)
Sircar, S.: Estimation of isosteric heats of adsorption of single gas and multicomponent gas-mixtures. Ind. Eng. Chem. Res. 31(7), 1813–1819 (1992). https://doi.org/10.1021/ie00007a030
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11(1), 121–127 (1965). https://doi.org/10.1002/aic.690110125
Siperstein, F.R., Myers, A.L.: Mixed-gas adsorption. AIChE J. 47(5), 1141–1159 (2001). https://doi.org/10.1002/aic.690470520
Sundaram, N.: A modification of the Dubinin isotherm. Langmuir 9(6), 1568–1573 (2002). https://doi.org/10.1021/la00030a024
Hamid, U., Vyawahare, P., Tun, H., Chen, C.-C.: Generalization of thermodynamic Langmuir isotherm for mixed-gas adsorption equilibria. AIChE J. 68(6), e17663 (2022). https://doi.org/10.1002/aic.17663
Builes, S., Sandler, S.I., Xiong, R.: Isosteric heats of gas and liquid adsorption. Langmuir 29(33), 10416–10422 (2013). https://doi.org/10.1021/la401035p
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14(1), 135–144 (1968). https://doi.org/10.1002/aic.690140124
Tun, H.: Prediction of Mixed-Gas Adsorption Equilibria and Isosteric Heat of Adsorption from Its Pure Component Adsorption Isotherms. Texas Tech University, Lubbock (2020)
Britt, H.I., Luecke, R.H.: The estimation of parameters in nonlinear implicit models. Technometrics 15(2), 233–247 (1973). https://doi.org/10.1080/00401706.1973.10489037
Cmarik, G., Son, K., Knox, J.: Standard Isotherm Fit Information for Dry CO2 on Sorbents for 4-Bed Molecular Sieve. 2017. NASA/TM—2017–219847. December 2017.
Szepesy, L., Illes, V.: Adsorption of gases and gas mixtures, I. Acta Chim. Hung. 35, 37–50 (1963)
Khvoshchev, S.S., Zverev, A.V.: Calorimetric study of NH3 and CO2 adsorption on synthetic faujasites with Ca2+, Mg2+, and La3+ cations. J. Colloid Interface Sci. 144(2), 571–578 (1991). https://doi.org/10.1016/0021-9797(91)90422-5
Llewellyn, P.L., Maurin, G.: Gas adsorption microcalorimetry and modelling to characterise zeolites and related materials. Comptes Rendus Chimie. Mar-Apr 8(3–4), 283–302 (2005). https://doi.org/10.1016/j.crci.2004.11.004
Sircar, S., Cao, D.V.: Heat of adsorption. Chem. Eng. Technol. 25(10), 945–948 (2002). https://doi.org/10.1002/1521-4125(20021008)25:10%3c945::Aid-ceat945%3e3.0.Co;2-f
Cao, D.V., Sircar, S.: Heats of adsorption of pure SF6 and CO2 on silicalite pellets with alumina binder. Ind. Eng. Chem. Res. 40(1), 156–162 (2001). https://doi.org/10.1021/ie000650b
Szepesy, L., Illes, V.: Adsorption of gases and gas mixtures, III. Acta Chim. Hung. 35, 245–253 (1963)
Dunne, J.A., Rao, M., Sircar, S., Gorte, R.J., Myers, A.L.: Calorimetric heats of adsorption and adsorption isotherms. 3. Mixtures of CH4 and C2H6 in Silicalite and Mixtures of CO2 and C2H6 in NaX. Langmuir 13(16), 4333–4341 (1997). https://doi.org/10.1021/la960984z
Sing, K.S.W.: Assessment of surface area by gas adsorption. In: Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G. (eds.) Adsorption by Powders and Porous Solids, pp. 237–268. Academic Press, Boca Raton (2014)
McClellan, A.L., Harnsberger, H.F.: Cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Interface Sci. 23(4), 577–599 (1967). https://doi.org/10.1016/0021-9797(67)90204-4
Livingston, H.K.: The cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Sci. 4(5), 447–458 (1949). https://doi.org/10.1016/0095-8522(49)90043-4
Acknowledgements
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Funding
Funding support is provided by the U. S. Department of Energy under the grant DE-EE0007888. The authors gratefully acknowledge the financial support of the Jack Maddox Distinguished Engineering Chair Professorship in Sustainable Energy sponsored by the J.F Maddox Foundation.
Author information
Authors and Affiliations
Contributions
UH: conceptualization-equal, data curation-lead, formal analysis-lead, investigation-lead, methodology-equal, software-lead, validation-lead, visualization-lead, writing-original draft-lead. PV: conceptualization-supporting, data curation-supporting, formal analysis-supporting, methodology-supporting, writing-original draft-supporting. C-CC: conceptualization-equal, data curation-supporting, formal analysis-supporting, funding acquisition-lead, investigation-lead, methodology-equal, project administration-lead, resources-lead, software-supporting, supervision-lead, validation-equal, visualization-supporting, writing-original draft-supporting, writing-review & editing-lead.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamid, U., Vyawahare, P. & Chen, CC. Estimation of isosteric heat of adsorption from generalized Langmuir isotherm. Adsorption 29, 45–64 (2023). https://doi.org/10.1007/s10450-023-00379-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00379-x