Abstract
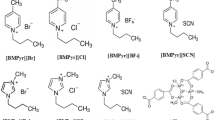
The potential advantage of using supported ionic liquids (SILs) for CO2 capture applications has been investigated in this work. The impregnation of 1-ethyl-3-methylimidazolium Acetate [emim][Ac] into two voluminous metal–organic frameworks (MOF-177 and MIL-101) is evaluated using different synthesis methods. The performance of the composite sorbents for CO2 capture has been evaluated using various characterization techniques. The successful incorporation of [emim][Ac] into the pores of MOF-177 and MIL-101 have been confirmed using thermogravimetric analysis and Fourier transform infrared spectroscopy results. Furthermore, the porosity of the as-synthesized samples using different synthesis methods have been measured using N2 adsorption experiments to evaluate the changes in the specific surface areas and pore volumes upon the introduction of [emim][Ac] to the support materials. A significant decrease in the porosity was realized for the [emim][Ac]-confined samples especially when using wet impregnation synthesis method compared to the dry mixing approach. The crystal structures of the MOF-177 and MIL-101 were found to be maintained after the synthetic steps, with some reductions in the X-ray diffraction peak intensities. No improvements in the CO2 uptakes could be achieved for the MIL-101 samples using both synthesis strategies, whereas the [emim][Ac]@MOF-177 samples prepared using wet impregnation method, has shown a remarkable enhancement in the CO2 capacity up to 0.3 mmol/g at 0.15 bar and 303 K. Moreover, the adsorption kinetics in MOF-177 based samples was found to be significantly fast as depicted from the first order rate constant values. The [emim][Ac]@MOF-177 samples exhibited a substantially stable cyclic adsorption–desorption performance up to 10 successive cycles with a considerably fast desorption kinetics.















Similar content being viewed by others
References
Álvarez, J.R., Sánchez-González, E., Pérez, E., Schneider-Revueltas, E., Martínez, A., Tejeda-Cruz, A., Islas-Jácome, A., González-Zamora, E., Ibarra, I.A.: Structure stability of HKUST-1 towards water and ethanol and their effect on its CO2 capture properties. Dalton Trans. 46, 9192–9200 (2017)
Babucci, M., Akçay, A., Balci, V., Uzun, A.: Thermal stability limits of imidazolium ionic liquids immobilized on metal-oxides. Langmuir 31, 9163–9176 (2015)
Babucci, M., Balci, V., Akçay, A., Uzun, A.: Interactions of [BMIM][BF4] with metal oxides and their consequences on stability limits. J. Phys. Chem. C 120, 20089–20102 (2016)
Carvalho, P.J., Álvarez, V.H., Schröder, B., Gil, A.M., Marrucho, I.M., Aznar, M., Santos, L.M., Coutinho, J.A.: Specific solvation interactions of CO2 on acetate and trifluoroacetate imidazolium based ionic liquids at high pressures. J. Phys. Chem. B 113, 6803–6812 (2009)
Chen, C., Feng, N., Guo, Q., Li, Z., Li, X., Ding, J., Wang, L., Wan, H., Guan, G.: Surface engineering of a chromium metal-organic framework with bifunctional ionic liquids for selective CO2 adsorption: synergistic effect between multiple active sites. J. Colloid Interface Sci. 521, 91–101 (2018)
Gray, M.L., Hoffman, J.S., Hreha, D.C., Fauth, D.J., Hedges, S.W., Champagne, K.J., Pennline, H.W.: Parametric study of solid amine sorbents for the capture of carbon dioxide. Energy Fuels 23, 4840–4844 (2009)
Hafizovic, J., Bjørgen, M., Olsbye, U., Dietzel, P.D.C., Bordiga, S., Prestipino, C., Lamberti, C., Lillerud, K.P.: The Inconsistency in adsorption properties and powder XRD Data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 129, 3612–3620 (2007)
Hasib-ur-Rahman, M., Siaj, M., Larachi, F.: Ionic liquids for CO2 capture—development and progress. Chem. Eng. Process. 49, 313–322 (2010)
Kinik, F.P., Altintas, C., Balci, V., Koyuturk, B., Uzun, A., Keskin, S.: [BMIM][PF6] incorporation doubles CO2 selectivity of ZIF-8: elucidation of interactions and their consequences on performance. ACS Appl. Mater. Interfaces 8, 30992–31005 (2016)
Koyuturk, B., Altintas, C., Kinik, F.P., Keskin, S., Uzun, A.: Improving gas separation performance of ZIF-8 by [BMIM][BF4] incorporation: interactions and their consequences on performance. J. Phys. Chem. C 121, 10370–10381 (2017)
Krupiczka, R., Rotkegel, A., Ziobrowski, Z.: Comparative study of CO2 absorption in packed column using imidazolium based ionic liquids and MEA solution. Sep. Purif. Technol. 149, 228–236 (2015)
Li, X., Cheng, F., Zhang, S., Chen, J.: Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 160, 542–547 (2006)
Lin, Y., Yan, Q., Kong, C., Chen, L.: Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 3, 1859 (2013)
Liu, A.-H., Ma, R., Song, C., Yang, Z.-Z., Yu, A., Cai, Y., He, L.-N., Zhao, Y.-N., Yu, B., Song, Q.-W.: Equimolar CO2 capture by N-substituted amino acid salts and subsequent conversion. Angew. Chem. Int. Ed. 51, 11306–11310 (2012)
Llewellyn, P.L., Bourrelly, S., Serre, C., Vimont, A., Daturi, M., Hamon, L., De Weireld, G., Chang, J.-S., Hong, D.-Y., Kyu Hwang, Y., Hwa Jhung, S., Férey, G.: High uptakes of CO2 and CH4 in mesoporous metal–organic frameworks MIL-100 and MIL-101, Langmuir, 24, 7245–7250 (2008)
Luo, Q., Song, X., Ji, M., Park, S.-E., Hao, C., Li, Y.: Molecular size- and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst. Appl. Catal., A 478, 81–90 (2014)
Mason, J.A., Sumida, K., Herm, Z.R., Krishna, R., Long, J.R.: Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 4, 3030–3040 (2011)
Millward, A.R., Yaghi, O.M.: Metal–organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 127, 17998–17999 (2005)
Mohamedali, M., Nath, D., Ibrahim, H., Henni, A.: Review of recent developments in CO2 capture using solid materials: metal organic frameworks (MOFs). In: Moya, B.L., Pous, J. (eds.) Greenhouse Gases. InTech, Rijeka (2016)
Mohamedali, M., Ibrahim, H., Henni, A.: Application of metal–organic frameworks (MOFs) for CO2 separation. In: García, S.N.H. (ed.) Metal-Organic Frameworks, pp. 123–161. Wiley, Weinheim (2018a)
Mohamedali, M., Ibrahim, H., Henni, A.: Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture. Chem. Eng. J. 334, 817–828 (2018b)
Moya, C., Alonso-Morales, N., Gilarranz, M.A., Rodriguez, J.J., Palomar, J.: Encapsulated ionic liquids for CO2 capture: using 1-butyl-methylimidazolium acetate for quick and reversible CO2 chemical absorption. ChemPhysChem, 17 (2016) 3891–3899
Nejat, P., Jomehzadeh, F., Taheri, M.M., Gohari, M., Abd, M.Z.. Majid: A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 43, 843–862 (2015)
Niu, M., Yang, H., Zhang, X., Wang, Y., Tang, A.: Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces 8, 17312–17320 (2016)
Praetorius, B., Schumacher, K.: Greenhouse gas mitigation in a carbon constrained world: The role of carbon capture and storage. Energy Policy 37, 5081–5093 (2009)
Ramdin, M., de Loos, T.W., Vlugt, T.J.H.: State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 51, 8149–8177 (2012)
Rao, A.B., Rubin, E.S., Technical, A.: Economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 36, 4467–4475 (2002)
Rochelle, G.T.: Amine scrubbing for CO2 capture. Science 325, 1652–1654 (2009)
Saha, D., Deng, S.: Structural stability of metal organic framework MOF-177. J. Phys. Chem. Lett. 1, 73–78 (2010)
Sun, X.-L., Deng, W.-H., Chen, H., Han, H.-L., Taylor, J.M., Wan, C.-Q., Xu, G.: A metal–organic framework impregnated with a binary ionic liquid for safe proton conduction above 100 °C. Chemistry A 23, 1248–1252 (2017)
Valkenberg, M.H., deCastro, C., Holderich, W.F.: Immobilisation of ionic liquids on solid supports. Green Chem. 4, 88–93 (2002)
Villanueva, M., Coronas, A., García, J., Salgado, J.: Thermal stability of ionic liquids for their application as new absorbents. Ind. Eng. Chem. Res. 52, 15718–15727 (2013)
Wang, X., Akhmedov, N.G., Duan, Y., Luebke, D., Li, B.: Immobilization of amino acid ionic liquids into nanoporous microspheres as robust sorbents for CO2 capture. J. Mater. Chem. A 1, 2978–2982 (2013)
Wang, J., Xie, D., Zhang, Z., Yang, Q., Xing, H., Yang, Y., Ren, Q., Bao, Z.: Efficient adsorption separation of acetylene and ethylene via supported ionic liquid on metal-organic framework. AlChE J. 63, 2165–2175 (2017)
Xue, W., Li, Z., Huang, H., Yang, Q., Liu, D., Xu, Q., Zhong, C.: Effects of ionic liquid dispersion in metal-organic frameworks and covalent organic frameworks on CO2 capture: a computational study. Chem. Eng. Sci. 140, 1–9 (2016)
Zeeshan, M., Keskin, S., Uzun, A., Enhancing CO2/CH4 and CO2/N2 separation performances of ZIF-8 by post-synthesis modification with [BMIM][SCN]. Polyhedron 155, 485–492 (2018)
Zhao, T., Jeremias, F., Boldog, I., Nguyen, B., Henninger, S.K., Janiak, C.: High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 44, 16791–16801 (2015)
Zhu, J., He, B., Huang, J., Li, C., Ren, T.: Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids. Microporous Mesoporous Mater. 260, 190–200 (2018)
Zoubeik, M., Mohamedali, M., Henni, A.: Experimental solubility and thermodynamic modeling of CO2 in four new imidazolium and pyridinium-based ionic liquids. Fluid Phase Equilib. 419, 67–74 (2016)
Acknowledgements
The authors would like to acknowledge the Faculty of Graduate Studies and Research (FGSR) at the University of Regina for the financial support in the form of a Graduate Research Fellowship (GRF). Acknowledgments are also due to the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), and the Clean Energy Technologies Research Institute (CETRi) at the University of Regina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamedali, M., Henni, A. & Ibrahim, H. Investigation of CO2 capture using acetate-based ionic liquids incorporated into exceptionally porous metal–organic frameworks. Adsorption 25, 675–692 (2019). https://doi.org/10.1007/s10450-019-00073-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00073-x