Abstract
Erosion, which is considered to be global problem, is the process of soil environment degradation due to the impact of wind (soil blowing, deflation) and water (soil washing, deep erosion). This leads to destruction of its structure, nutrients removal and finally bedrock exposition and desertification. One of the ways to prevent this phenomenon is the soil conditioners usage. These substances, also called soil flocculants, are macromolecular compounds which contribute to the reinforcement of soil structure as a result of its adsorption on the mineral surface. The soil conditioners commonly used in agriculture is ionic polyacrylamide (PAM)—both anionic and cationic. These macromolecular substances affect soil surface properties and adsorption behaviour of substances present in the environment—nutrients, organic molecules and also hazardous compounds as well as toxic heavy metals (i.e. chromium(VI) ions). The aim of this study was to investigate the impact of solution pH, type of ionic groups in the polyacrylamide macromolecules and chromium(VI) ion concentration on the adsorption mechanism of Cr(VI) and PAM on the montmorillonite surface. The adsorption and electrokinetic properties of the montmorillonite—AN PAM or CT PAM/Cr(VI) systems were examined by means of spectrophotometry, potentiometric titration, SEM, XRD and DRS methods. It was shown that in the adsorbed layer composed of PAM-Cr(VI) complexes reduction of Cr(VI) to Cr(III) takes place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dispersed colloidal particles of various minerals form the so-called soil sorption complex. Silicates and products of their transformation—aluminosilicates form the main mineral component of soils. They are formed as a result of silicon, oxygen, metal ions, as well as water molecules binding. The structural unit of silicates is the silicate anion—SiO44− (Handke 2005; Sarbak 2009) A characteristic property of these minerals is the ability to exchange silicon atoms for aluminum ones in their crystal networks. As a result of this isomorphic substitution, minerals with an aluminum atom in the center of the tetrahedron (aluminosilicates) are created. Clay minerals which are hydrated aluminosilicates have a characteristic layered structure. Depending on the relative location of tetrahedral and octahedral units these minerals can be divided into the layered 2:1, 1:1 or amorphous aluminosilicates (Kunert and Zaborski 2006).
Montmorillonite is a representative of the layered 2:1 clay mineral with one alumina octahedral sheet sandwiched between two silica tetrahedral sheets (Kurleto at al. 2015, Mikuła and Łach 2002; Kacperski 2002). A small electric charge in the structure of 2:1 clay minerals (mainly between layers) is compensated by the so-called inter-layered cations (e.g. hydrogen, sodium, calcium or magnesium cations). A characteristic feature of three-layered aluminosilicates is the ability to absorb water molecules or organic compounds, as well as the capability of ion exchange. These properties are due to the presence of weak van der Waals forces between packages. Absorption of molecules or ions, also known as intercalation, causes the expansion of the space between the silica and alumina sheets. This phenomenon also known as intercalation can be affected by many factors such as pressure, temperature or humidity of the environment, as well as intercalate concentration and structure (Sarbak 2010; Olejnik 2008; Natkański et al. 2012; Uddin 2017; Zhu et al. 2016).
Soils rich in aluminosilicate fractions such as montmorillonite, are naturally exposed to degrading factors such as wind or water. They can cause erosion, involving the destruction of soil structure and reduction of its fertility. This leads to environment destruction and soil nutrients removal, the bedrock exposition and desertification (Bronick and Lal 2005; Sojka et al. 2007; Lee et al. 2013) One of the ways to prevent soil erosion is the addition of soil conditioners (e.g. polyacrylamide or poly(acrylic acid)). These substances promote strengthening of the structure and stability of the soil by the flocculation phenomenon. Soil flocculants are macromolecular compounds which can bind loose mineral particles into larger aggregates by the polymer bridges formation between them (Mclaughlin and Bartholomew 2007; Basaran and Tasdemir 2014; Graveling et al. 1997; Lee and Schlautman 2015; Deng et al. 2006; Guezennec et al. 2015; He et al. 2017). Moreover, water and air relations or soil structure improvement in the presence of soil conditioners are observed. The presence of polymer affects the increase of water infiltration into the soil and can affect changes in surface properties of mineral particles (i.e. behaviour of nutrients, toxic heavy metals, organic substances present in the environment) (Mamedov et al. 2010; Green et al. 2004; Ajwa and Trout 2006; Wang et al. 2016; Lee et al. 2014; Ben-Hur and Keren 1997).
Environmental pollution with heavy metals has increased with the development of civilization. The main sources of environmental contamination are industrial wastes and sewages, metallurgical and chemical industry, mining or transport emissions. Also mineral fertilizers and products for plant protection can contribute to pollution of soil and water (Ociepa-Kubicka and Ociepa 2012; Sas-Nowosielska 2009, Vasquez-Murrieta et al. 2006). The concentration of heavy metals in the environment can be various and depends on their physicochemical properties and the ability to form soluble complexes (Nosal-Wiercińska and Dalmata 2002; Nosal-Wiercińska 2014; Grochowski et al. 2016). Depending on type, chemical form or dosage heavy metals can have a stimulating or negative effect on the living organisms. The elements such as Fe, Mn, Cu, Mo, Zn are the examples of metals which in a low amount are necessary for the proper course of metabolic processes, and in a greater concentration have harmful and toxic effects (Wang et al. 2003; Kowalski 1994; Devi and Fingermann 1995; Quek et al. 1998, Pueyo et al. 2004; Wójcik et al. 2004).
Chromium is one of the heavy metals. In nature this element has two stable oxidation levels and occurs as Cr(III) and Cr(VI). However, physicochemical properties and toxicity of these forms are different. Chromium(III) plays a key role in the metabolism of glucose and certain fats as well as proteins in mammals. Because of its strong oxidative properties chromium(VI) causes oxidation of unsaturated bonds in proteins (including enzymes), fatty and nucleic acids. This heavy metal ion shows high toxicity (i.e. mutagenic, carcinogenic and teratogenic properties) to humans, animals and plants. Damage of photosynthetic apparatus or gastrointestinal tract, skin changes and allergies are caused by chromium(VI) (Szymański 2009; Karthikeyan et al. 2005; Stearns et al. 1995; Ociepa-Kubicka and Ociepa 2012; Hadjispyrou et al. 2001; Zhang et al. 2012).
The main aim of the present study is the comparison of adsorption mechanism of two forms of ionic polyacrylamide (anionic and cationic) on the montmorylonite surface. The efficiency of chromium(VI) ions accumulation in the polymeric adsorption layer was also investigated. Additionally, the possibility of Cr(VI) reduction to non-toxic Cr(III) within the polymer-heavy metal ions complexes formed on the soil mineral surface was examined.
2 Experimental
2.1 Materials and their characteristics
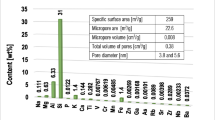
Montmorillonite—2:1 aluminosilicate (Sigma-Aldrich) was used as an adsorbent in the experiments. Specific surface area and its porosity were determined using the nitrogen adsorption/desorption method (Micromeritics ASAP 2020 analyzer). The elemental composition of the mineral was determined by the XRF technique (Panalytical ED-XRF type Epsilon 3 spectrometer). Montmorillonite textural properties and elemental composition of the adsorbent were described in our previous paper (Wiśniewska et al. 2018). Using the high resolution scanning electron microscope Quanta 3D FEG (FEI, Field Electron and Ion Co.) the SEM images of clay mineral without or with the flocculant were obtained. To determine the possibility of intercalation process occurrence in montmorillonite after polymer addition the XRD technique was applied (diffractometer Empyrean, PANalytical).
Two types of polyacrylamide (Korona) differing in the ionic group contents were applied as the adsorbate in the study. The first one—anionic polyacrylamide (AN PAM) contained 30% of the ionizable carboxyl groups whereas the cationic polyacrylamide (CT PAM)—35% of the quaternary amine groups. The average molecular weight of the adsorbate was equal to 13,000 and 7000 kDa, respectively. The dissociation degree of carboxyl groups in the PAM macromolecules was determined by means of the potentiometric titration method using the procedure presented in our previous papers (Wiśniewska et al. 2015, 2016). The detailed characteristics of the polymer samples are presented in Table 1.
2.2 Spectrophotometric measurements
The adsorption measurements were performed at 25 °C with the two solution pH values (neutral and slightly acidic conditions typical of clay soils), i.e. 5 and 7 ± 0.1. The sodium chloride (NaCl) solution with the concentration of 0.001 mol/L was used as a supporting electrolyte. Adsorbed amounts of polymer and heavy metal ion on the mineral surface were determined spectrophotometrically using the UV–Vis spectrophotometer (Carry 100; Varian) by the static method based on the difference between the PAM or Cr(VI) concentrations in the solutions before and after the adsorption. For determination of anionic polyacrylamide concentration in the solution the reaction of polyacrylamide with hyamine proposed by Crummet and Hummel (1963) was applied. It is based on the spectrophotometric measurements of absorbance generated by the PAM reaction with hyamine 1622 (N-benzyl-N,N-dimethyl-2-{2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy}ethanaminiumchloride). The absorbance originating from the white colour of solution, indicating PAM-hyamine complex formation, was measured at the wavelength 500 nm after 15 min of the hyamine addition. The cationic polyacrylamide concentration was determined spectrophotometrically using the brilliant yellow as the indicator. After adjusting pH 9, 0.5 ml of the probe was added to 4.5 ml of the indicator, and absorbance was measured at 495 nm. In the case of chromium(VI), the reaction with diphenylcarbazide was used (Sas-Nowosielska 2009). In the acidic environment Cr(VI) oxidizes 1,5-diphenylcarbazide(I) to diphenylcarbazone(II) whereas it itself is reduced to Cr(III). The absorbance of the obtained purple complex between Cr(III) and diphenylcarbazone(II) was measured at a wavelength of 546 nm (Gardner and Comber 2002). Adsorption process was carried out under the conditions of continuous shaking (water bath OLS 200, Grant) for 24 h. The montmorillonite weight in each examined system was 0.003 g. The appropriate pH values of the examined suspensions were adjusted with a pH-meter (Beckman Instruments). The solids were centrifuged using a microcentrifuge (MPW Med. Instruments) and the clear solutions were collected for further analysis of polymer and Cr(VI) ion concentration.
The changes in the soil suspension stability without and with the polymer were monitored using the spectrophotometry (spectrophotometer Carry 1000, Varian). The absorbance of montmorillonite suspension with and without polyacrylamide was measured in a function of time at the wavelength λ = 500 nm.
2.3 Potentiometric titrations
Using the potentiometric titration method surface charge density as a function of solution pH and point of zero charge (pzc) were determined. The potentiometric titrations of montmorillonite suspensions in the absence and presence of polyacrylamide and/or Cr(VI) were performed in the thermostated Teflon vessel using 0.1 g of the mineral. The solid surface charge density was calculated with the special program Titr_v3 (authored by Janusz 1994) using the following equation:
where ΔV—the difference in the base volume added to a suspension and a supporting electrolyte solution that leads to the specific pH value, c—the base concentration, F—the Faraday constant, m—the solid mass in the suspension, S—the solid surface area. The measuring set was composed of the following parts: burette Dosimat 665 (Methrom), thermostat RE204 (Lauda), pH meter 71 (Beckman), computer and printer.
2.4 Spectroscopic measurements
To determine the reduction process of Cr(VI) to Cr(III) occurring in the adsorption layer the Diffuse Reflectance Spectroscopy (DRS) was applied. After the adsorption process, the solid samples were centrifuged, dried and exposed to the IR beam in the course of DRS measurements. The DRS spectra were measured using the Jasco V-660 spectrometer equipped with a diffuse reflectance attachment PIV-756 (Jasco, Japan). In order to separate the signals coming from various forms of chromium, the procedure of deconvolution of the obtained spectra was applied.
3 Results and discussion
3.1 Adsorbent characteristic
Specific surface area and porosity of montmorillonite were determined using a low-temperature nitrogen adsorption/desorption method. The obtained results were presented in our previous paper (Wiśniewska et al. 2018). The specific surface area of the examined 2:1 type aluminosilicate is equal to 259 m2/g. It is associated with the specific layered structure of this clay mineral. The observed isotherms of IV type with the H3 hysteresis loop indicate a multilayer adsorption on the mesopores (Anovitz and Cole 2015). Moreover, there are two groups of mesopores: the first of the average diameter equal to 3.8 nm and the other—5.6 nm. The pore volume of the soil mineral is about 0.37 cm3/g.
The SEM images of montmorillonite with or without anionic/cationic polyacrylamide (Fig. 1) show the PAM adsorption effect on the mineral aggregation. Figure 1a presents highly dispersed solid particles without the polymer. Both the anionic and cationic PAM addition causes aggregation of solid particles but the flocks formed in the cationic soil flocculant presence are significantly larger.
3.2 Stability studies
The addition of polyacrylamide changes considerably the montmorillonite suspension stability (Fig. 2). In the case of CT PAM presence, the system shows small absorbance values after only 5 min from the beginning of measurements, which indicates formation of large flocks sedimenting easily on the bottom of the measuring vial. Thus, CT PAM exhibits flocculating properties with respect to the soil mineral particles. The stability properties in systems containing CT PAM and Cr(VI) ions changes minimally (in comparison to suspensions without Cr(VI)), both at pH 5 and 7.
In turn, in the anionic polymer presence the absorbance remains at a high level throughout the experiment (especially at pH 5). At pH 5 there is the point of zero charge of montmorillonite and adsorption of anionic polyacrylamide chains (whose carboxyl groups are totally dissociated, Table 1) can lead to electrosteric interactions between the solid particles covered with the AN PAM layers. But the greatest effect of montmorillonite suspension destabilization (among all examined systems) was obtained after addition of AN PAM and Cr(VI) ions at pH 7.
3.3 Adsorption of polyacrylamide and Cr(VI) on the montmorillonite surface
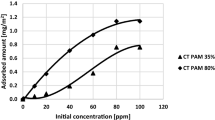
The ionic polyacrylamide and Cr(VI) ions amounts adsorbed on the montmorillonite surface are presented in Figs. 3 and 4. These values depend largely on the solution pH. The adsorbed amounts of cationic PAM increase with the pH decrease (higher adsorption of CT PAM is observed at pH 7 than 5). The pKb value for the cationic polyacrylamide is 9.3. At pH 5 and 7 the degree of quaternary amine groups dissociation in the polymer chains varies in the range of 99.4–99.9%. Thus, all cationic groups of the polymer are present in the ionized form and they are a source of positive charge of adsorbing macromolecules. The higher adsorption affinity of CT PAM at pH 7 is due to favourable electrostatic attraction between the positively charged macromolecules and the negatively charged solid surface. In the case of anionic polyacrylamide, the adsorbed amount decreases with the increasing pH. The pKa value of anionic PAM is equal to 3.2 and in the studied pH range the degree of carboxyl groups dissociation is from 98.7 to 99.8%. Therefore smaller adsorption of AN PAM at pH 7 is caused by unfavorable electrostatic repulsion between the montmorillonite surface and the negatively charged ionized –COOH groups (present in macromolecules) and negatively charged solid surface. Under such electrostatic conditions adsorption of anionic polyacrylamide on the montmorillonite surface proceeds mainly through hydrogen bonds which are formed between the solid hydroxyl groups and the polymer functional groups (occurring both in the undissociated and dissociated forms). Moreover, polyacrylamide characterized by a higher content of cationic groups having a quaternary amine groups shows a noticeably higher adsorption level in comparison to the PAM containing ionizable carboxyl groups. At pH about 5 the total charge of montmorillonite surface is equal to zero (point of zero charge) and under such conditions the solid surface is neutral. For this reason at higher values of pH the electrostatic forces are more beneficial for the cationic polymer adsorption at pH 7 whereas in the case of anionic polyacrylamide the adsorbed amount increases at pH 5.
The adsorption of chromium(VI) ions decreases with the increasing pH value. These toxic metal ions occur in the forms of CrO42− and HCrO4− in the range of studied pH values. Therefore their affinity for the montmorillonite surface decreases with the increase of solid negative charge (similarly to negatively charged macromolecules of anionic polymer).
The polymer (and Cr(VI) ions) present in the montmorillonite system does not cause the changes in the crystal structure of montmorillonite. The ionic polyacrylamide chains are probably too large to penetrate the space between the silica and alumina sheets of solid mineral and the intercalation process does not take place. Such behaviour was confirmed by XRD measurements. The analysis of their results indicated only minimal changes in basal spacing d001 (Frost and Rintoul 1996; Krupskaya et al. 2017), obtained at values of 2θ angle changing in the range 6–6.5, for the montmorillonite particles modified with ionic polyacrylamide and Cr(VI) ions (Table 2) in comparison to the unmodified solid.
3.4 Effects of Cr(VI) concentration on the polyacrylamide adsorbed amount on the montmorillonite surface
As can be seen in Fig. 5 in the systems of mixed adsorbates, the Cr(VI) ions concentration has a minimal effect on the cationic polyacrylamide adsorption. In the case of AN PAM 30% the addition of Cr(VI) ions of the concentration 1 ppm evidently increases the adsorption level of ionic polyacrylamide whereas the presence of chromium(VI) ions of the concentrations of 10 and 100 ppm does not affect the amount of adsorbed AN PAM 30%. Such behaviour is associated with creation of hydrogen bonds between the PAM neutral amide groups and CrO42− and HCrO4− ions (Bajpai and Johnson 2007; Zhang et al. 2012). Consequently, the polymeric macromolecules assume the specific conformation at the solid/liquid interface which directly determines the polymer adsorbed amount.
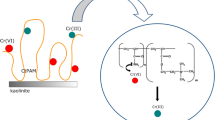
On the other hand, the cationic polyacrylamide has a strongly developed conformation due to the presence of positively charged groups in the pH range 5–7. Due to the electrostatic attraction between the CrO42− and HCrO4− anions and the cationic polymer quaternary amine groups, as well as formation of hydrogen bonds between the PAM amide functional groups and chromium(VI) ions, a slight increase in the adsorbed amount of cationic polyacrylamide in the presence of toxic metal ions is observed (Bajpai and Johnson 2007; Zhang et al. 2012). The schematic representation of adsorption mechanism of PAM-Cr(VI) complexes on the montmorillonite surface was presented in Fig. 6.
The schematic representation of adsorption mechanism of PAM-Cr(VI) complexes on the montmorillonite surface (schema of montmorillonite structure: http://www.bentonites.net, Ubowska 2010)
3.5 Influence of PAM presence and Cr(VI) concentration on the Cr(VI) adsorbed amount on the montmorillonite surface
At the lowest examined Cr(VI) ions concentration (i.e. 1 ppm), both anionic and cationic polyacrylamide presence does not affect the adsorption amount of heavy metal ions (Fig. 7a, b). In this case all chromium(VI) ions undergo adsorption on the solid surface. However, with the increase of Cr(VI) ions concentration in the solution, its adsorption in the PAM presence also increases. In the system with the Cr(VI) ions concentration equal to 100 ppm the adsorption of these toxic metal ions in the presence of both ionic forms of PAM is over twice higher in comparison to the system without polymer. The CT PAM chains are endowed with a positive charge in the investigated pH range, which promotes adsorption of negatively charged chromium(VI) anions. On the other hand, the anionic polyacrylamide adsorbed on the montmorillonite surface captures CrO42− and HCrO4− ions from the solution effectively through hydrogen bond creation. As a consequence, a significant increase in the Cr(VI) ions adsorption is observed in the ionic polyacrylamide presence.
3.6 Electrokinetic properties of montmorillonite particles without and with polyacrylamide and Cr(VI)
Table 3 shows the pHpzc of the examined systems. At pH 5.02 the total charge of montmorillonite surface is equal to zero—the concentration of the positively (–SiOH2+) and negatively (–SiO−) charged surface groups is the same (pzc conditions). At pH values lower than pHpzc the solid surface is positively charged and above the pHpzc value—negatively charged. The CT PAM addition causes the increase of the solid surface charge density compared to the system without the polymer. Besides the unionizable amide groups cationic polyacrylamide contains 35% of quaternary amine groups which are a source of positive charge of the polymer chains. Under the examined pH conditions positively charged groups are very numerous and their adsorption on the mineral surface causes a significant increase of its surface charge density. However, only a part of –N(CH3)3+ groups is located directly on the solid surface when most of them are placed in the by-surface layer of the solution in the tail and loop structures of the adsorbed macromolecules causing an increase of pHpzc value. In the case of AN PAM/montmorillonite system the addition of anionic polymer causes a decrease of the solid surface charge density. The increase in the solution pH results in the complete dissociation of polymeric macromolecules and their negatively charged –COO− groups (mostly placed in the by-surface layer of the solution) cause a decrease of σ0 and pHpzc values.
The chromium(VI) ions adsorption on the montmorillonite surface causes shift of pHpzc to the value of about 6.5. Increase of this parameter is common behaviour observed in the case of small anion adsorption (such as chromium(VI) anions). Their interactions with the solid hydroxyl groups result in creation of the additional number of positively charged surface sites and the σ0 value increases (Janusz 1999; Wiśniewska et al. 2017). In the adsorption systems containing CT PAM and Cr(VI) the montmorillonite pHpzc parameter is similar to that of the polymer containing system. Thus, it can be stated that the polymer-metal complexes present in the interfacial layer assume such spatial arrangement that the chromium(VI) ions present in their structure cause only insignificant changes in the surface charge of the investigated mineral. In the case of AN PAM 30%, the increase of solid surface charge of the mixed system in comparison to the simple one (hydrogen bonds are formed between chromate anions and amide groups of polymer increasing the negative charge of PAM macromolecules) is observed (Bajpai and Johnson 2007; Zhang et al. 2012).
3.7 Reduction of Cr(VI) to Cr(III) in the montmorillonite—PAM system
Diffuse reflectance spectroscopy (DRS) allows identification of both chromium forms (on both the III and VI oxidation degrees) after their sorption in the montmorillonite-PAM system. Figure 8a and b presents obtained DRS spectra for the examined AN PAM and CT PAM containing systems, respectively. The region of DRS spectra related to the chromium(III) form is at 620 nm. The signals below 550 nm are due to the presence of chromium(VI). It can be concluded that chromium(VI) bound with ionic polyacrylamide in the adsorption layer on the montmorillonite surface undergoes reduction to the chromium(III) form. Free electron pairs located on the nitrogen atoms of PAM amide groups are probably involved in this process. This is a very desirable phenomenon due to non-toxic properties of chromium(III) form compared to chromium(VI) one.
4 Conclusions
Based on the obtained results there can be drawn the following conclusions:
-
(1)
Higher adsorption of polyacrylamide on the montmorillonite surface is observed in the case of the polymer with a quaternary amine groups due to the more favourable electrostatic attraction occurring between the positively charged macromolecules and the negatively charged surface of montmorillonite.
-
(2)
A larger amount of chromium(VI) ions is adsorbed at pH 5 because with the pH increase there are more and more negatively charged groups on the surface of aluminosilicate which is not favourable for the adsorption of chromium(VI) anions.
-
(3)
Anionic and cationic PAM captures CrO42− and HCrO4− ions effectively from the solution by electrostatic attraction between the chromium(VI) anion and the cationic polymer quaternary amine groups as well as formation of hydrogen bonds between the PAM amide groups and the chromium(VI) ions which contributes to a significant increase in their adsorption.
-
(4)
The presence of chromium(VI) ions and ionic polyacrylamide affects montmorillonite electrokinetic properties.
-
(5)
Both anionic and cationic polyacrylamide flocculants adsorbed on the montmorillonite particles can cumulate heavy metal ions.
-
(6)
In the examined montmorillonite-PAM-Cr systems there is observed the process of reduction of Cr(VI) to Cr(III) which is beneficial for the environment due to non-toxic properties of chromium(III).
References
Ajwa, H.A., Trout, T.J.: Polyacrylamide and water quality effects on infiltration in sandy loam soils. Soil Sci. Soc. Am. J. 70, 643–650 (2006)
Anovitz, L.M., Cole, D.R.: Characterization and analysis of porosity and pore structure. Rev. Min. Geochem. 80, 161–164 (2015)
Bajpai, S.K., Johnson, S.: Removal of Cr(VI) oxy-anions from aqueous solution by sorption into poly(acrylamide-co-maleic acid) hydrogels. Sep. Pur. Technol. 42, 1049–1064 (2007)
Basaran, H.K., Tasdemir, T.: Determination of flocculation characteristics of natural stone powder suspensions in the presence of different polymers. Physicochem. Probl. Miner. Proc. 50, 69–84 (2014)
Ben-Hur, M., Keren, R.: Polymer effects on water infiltration and soil aggregation. Soil Sci. Soci. Am. J. 61, 565–570 (1997)
Bronick, C.J., Lal, R.: Soil structure and management: a review. Geoderma 124, 3–22 (2005)
Crummett, W.B., Hummel, R.A.: The determination of traces of polyacrylamides in water. J. Am. Water Works Assoc. 1, 209–219 (1963)
Deng, Y., Dixon, J.B., White, G.N.: Adsorption of polyacrylamide on smectite, illite, and kaolinite. Soil Sci. Soci. Am. J. 70, 297–304 (2006)
Devi, M., Fingermann, M.: Inhibition of acetylcholinesterase activity in the central nervous system of the red swamp crayfish, procambarus clarkia, by mercury, cadmium and lead. Bull. Environ. Contam. Toxicol. 55, 746–750 (1995)
Frost, R.L., Rintoul, L.: Lattice vibration of montmorillonite: an FT Raman and X-ray diffraction study. Appl Clay Sci. 11, 171–183 (1996)
Gardner, M., Comber, S.: Determination of trace concentrations of hexavalent chromium. Analyst 127, 153–156 (2002)
Graveling, G.J., Ragnarsdottiv, K.V., Allen, G.C., Eastman, J., Brady, P.V., Balsley, S.D., Skuse, D.R.: Controls on polyacrylamide adsorption to quartz, kaolinite, and feldspar. Geochim. Cosmochim. Acta 61, 3515–3523 (1997)
Green, V.S., Scott, D.E., Graveel, J.G., Norton, L.D.: Stability analysis of soil aggregates treated with anionic polyacrylamides of different molecular formulations. Soil Sci. 169, 573–581 (2004)
Grochowski, M., Nosal-Wiercińska, A., Wiśniewska, M., Szabelska, A., Gołȩbiowska, B.: The effects of homocysteine protonation on double layer parameters at the electrode/chlorates (VII) interface, as well as the kinetics and the mechanism of Bi (III) ion electroreduction. Elecrochim. Acta 207, 48–57 (2016)
Guezennec, A.G., Michel, C., Bru, K., Touze, S., Desroche, N., Mnif, I., Motelica-Heino, M.: Transfer and degradation of polyacrylamide based flocculants in hydrosystems: a review. Environ. Sci. Pollut. Res. 22(9), 6390–6406 (2015)
Hadjispyrou, S., Kungolos, A., Anagnostopoulos, A.: Toxicity, bioaccumulation and interactive effects of organofin, cadmium, and chromium on Artemia franciscana. Ecotoxicol. Environ. Saf. 49, 179–186 (2001)
Handke, M.: Krystalochemia krzemianów. Uczelnianie Wydawnictwo Naukowo-Dydaktyczne, Kraków (2005)
He, J., Chu, J., Tan, S.K., Vu, T.T., Lam, K.P.: Sedimentation behavior of flocculant-treated soil slurry. Mar. Geores. Geotechnol. 35, 593–602 (2017)
Janusz, W.: Electrical double layer at the metal oxide/electrolyte interface in interfacial forces and fields: theory and applications in Surfactant science, chap. 4, vol. 85, Marcel Decker, New York (1994)
Janusz, W.: Electrical double layer at the metal oxide/electrolyte interface, in ‘interfacial forces and fields: theory and applications. M. Dekker, New York (1999)
Kacperski, M.: Polymer nanocomposites. Part I. General characteristics, fillers and nanocomposites based on termosetting polymers. Polimery 47, 801–807 (2002)
Karthikeyan, T., Rajgopal, S., Miranda, I.R.: Chromium(VI) adsorption from aqueous solution by Hevea brasilinesis sawdust activated carbon. J. Hazard. Mater. 124, 192–199 (2005)
Kowalski, Z.: Treatment of chromic tannery wastes. J. Hazard. Mater. 37, 137–144 (1994)
Krupskaya, V.V., Zakusin, S.V., Tyupina, E.A., Dorzhieva, O.V., Zhukhlistov, A.P., Belousov, P.E., Timofeeva, M.N.: Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 7, 49 (2017)
Kunert, A., Zaborski, M.: The structure, properties and uses of layered minerals. Przem. Chem. 85, 1510–1517 (2006)
Kurleto, Ż, Grabowska, B., Kaczmarska, K., Szymański, Ł: Wiązania chemiczne występujące w montmorylonicie. Archiv. Found. Eng. 15, 77–92 (2015)
Lee, B.J., Schlautman, M.A.: Effects of polymer molecular weight on adsorption and flocculation in aqueous kaolinite suspensions dosed with nonionic polyacrylamides. Water 7, 5896–5909 (2015)
Lee, S.S., Chang, S.X., Chang, Y.Y., Ok, Y.S.: Commercial versus synthesized polymers for soil erosion control and growth of Chinese cabbage. SpringerPlus 2, 1–10 (2013)
Lee, C.S., Robinson, J., Chong, M.F.: A review on application of flocculants in wastewater treatment. Proc. Saf. Environ. Protect. 92, 489–508 (2014)
Mamedov, A.J., Wagner, L.E., Huang, C., Norton, L.D., Levy, G.J.: Polyacrylamide effects on aggregate and structure stability of soils with different clay mineralogy. Soil Sci. Soc. Am. J. 74, 1–12 (2010)
Mclaughlin, R.A., Bartholomew, N.: Soil factors influencing suspended sediment flocculantion by polyacrylamide. Soil Sci. Am. J. 71, 537–544 (2007)
Mikuła, J., Łach, M.: Potencjalne zastosowania glinokrzemianów pochodzenia wulkanicznego. Mechanika Czasopismo Techniczne 109, 109–122 (2002)
Natkański, P., Białas, A., Kuśtowski, P.: Synteza kompozytów poli(kwas akrylowy)-bentonit oraz poliakryloamid-bentonit do zastosowań adsorpcyjnych. Chemik 66, 742–749 (2012)
Nosal-Wiercińska, A.: Intermolecular interactions in systems containing Bi(III)—ClO4 – H2O—selected amino acids in the aspect of catalysis of Bi(III) electroreduction. Electroanalysis 26, 1013–1023 (2014)
Nosal-Wiercińska, A., Dalmata, G.: Studies of the effect of thiourea on the electroreduction of In(III) ions in perchloric acid. Electroanalysis 14, 1275–1280 (2002)
Ociepa-Kubicka, A., Ociepa, E.: Toksyczne oddziaływanie metali ciężkich na rośliny, zwierzęta i ludzi. Inżynieria i Ochrona Środowiska 15, 169–180 (2012)
Olejnik, M.: Polymer nano-composites with montmorillonyte - obtaining, assesment methods, properties and applications. Techniczne Wyroby Włókiennicze 16, 67–74 (2008)
Pueyo, M., Lopez-Sanchez, J.F., Rauret, G.: Assessment of CaCl2, NaNO3, and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal. Chim. Acta 504, 217–225 (2004)
Quek, S.Y., Wase, D.A.J., Forster, C.F.: The use of Sago waste for the adsorption of lead and copper. Water S. A. 24(3), 251 (1998)
Sarbak, Z.: Nieorganiczne materiały nanoporowate. Wydawnictwo Naukowe UAM, Poznań (2009)
Sarbak, Z.: Application of sorbents in the process of soil remediation. Chemistry-Didactics-Ecology-Metrology 15, 77–92 (2010)
Sas-Nowosielska, A.: Fitotechnologie w remediacji terenów zanieczyszczonych przez przemysł cynkowo-ołowiowy, Monografia nr 189. Wyd. Politechniki Częstochowskiej, Częstochowa (2009)
Sojka, R.E., Bjorneberg, D.L., Entry, J.A., Lentz, R.D., Orts, W.J.: Polyacrylamide in agriculture and environmental land management. Agronomy 92, 75–162 (2007)
Stearns, D.M., Kennedy, L.J., Courtney, K.D., Giangrande, P.H., Phieffer, L.S., Wetterhahn, K.E.: Reduction of chromium (VI) by ascorbate leads to chromium—DNA binding and DNA strand breaks in vitro. Biochemistry 34, 910 (1995)
Szymański, K.: Związki ołowiu i chromu w środowisku naturalnym i odpadach. Rocznik Ochrona Środowiska 11, 173–181 (2009)
Ubowska, A.: Montmorillonite as a polyurethane foams flame retardant. Archiv. Combust. 30, 459–462 (2010)
Uddin, F.: Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 39, 2804–2814 (2008)
Uddin, M.K.: A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017)
Vasquez-Murrieta, M.S., Migules-Garduno, I., Franco-Hernandez, O., Govaerts, B., Dendooven, L.: C and N mineralization and microbial biomass in heavy-metal contaminated soil. Eur. J. Soil Biol. 42, 89–98 (2006)
Wang, Q., Cui, Y., Liu, X., Dong, Y., Christie, P.: Soil contamination and plant uptake of heavy metals at polluted sites in China. J. Environ. Sci. Health A 38, 823–838 (2003)
Wang, Z., Zhang, H., Pan, G.: Ecotoxicological assessment of flocculant modified soil for lake restoration using integrated biotic toxicity index. Water Res. 97, 133–141 (2016)
Wiśniewska, M., Chibowski, S., Urban, T.: Impact of polyacrylamide with different contents of carboxyl groups on the chromium(III) oxide adsorption properties in aqueous solution. J. Hazard. Mater. 283, 815–823 (2015)
Wiśniewska, M., Chibowski, S., Urban, T.: Nanozirconia surface modification by anionic polyacrylamide in relation to the solid suspension stability—effect of anionic surfactant addition. Powder Technol. 302, 357–362 (2016)
Wiśniewska, M., Chibowski, S., Urban, T.: Comparison of adsorption affinity of ionic polyacrylamide for the surfaces of selected metal oxides. Adsorp. Sci. Technol. 35, 582–591 (2017)
Wiśniewska, M., Fijałkowska, G., Szewczuk-Karpisz, K.: The mechanism of anionic polyacrylamide adsorption on the montmorillonite surface in the presence of Cr(VI) ions. Chemosphere 211, 524–534 (2018)
Wójcik, W., Odum, H.T., Szilder, Ł: Ołów i cynk w środowisku oraz rola mokradeł w ich usuwaniu. Uczelniane Wydawnictwa Naukowo-Dydaktyczne, Kraków (2004)
Zhang, D., Ma, Y., Feng, H., Hao, Y.: Adsorption of Cr(VI) from aqueous solution using carbon-microsilica composite adsorbent. J. Chil. Chem. Soc. 57, 964–968 (2012)
Zhu, R., Chen, Q., Zhou, Q., Xi, Y., Zhu, J., He, H.: Adsorbents based on montmorillonite for contaminant removal from water: a review. Appl. Clay Sci. 123, 239–258 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wiśniewska, M., Fijałkowska, G., Szewczuk-Karpisz, K. et al. Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium(VI) ions. Adsorption 25, 41–50 (2019). https://doi.org/10.1007/s10450-018-9990-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-018-9990-x