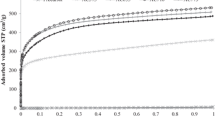
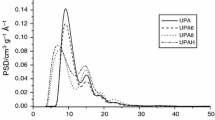
Abstract
Different techniques to prepare activated carbons exist. Most of them lead to materials with interesting properties, however, as far as we know, these features have been barely evaluated over time. In this context, the evolution of the textural and surface chemistry properties over time of different activated carbons is evaluated. For this purpose, six commercial and a homemade synthetized activated carbons with different characteristics were put in a climatic chamber for 22 months under constant conditions of temperature and relative humidity (T = 40 °C, RH = 80%). The samples were characterized at different periods of time by means of N2, CO2 and H2O adsorption measurements and by thermogravimetric analysis. The results show that contrary to what can be expected, the surface chemistry was not the only property that has undergone modifications but also the porous properties, and for some samples the latter was even very important. These changes were observed for all samples; however some of them are more affected like metal impregnated carbon and activated carbon fibers, whereas others seem to be more resistant to ageing such as chemically activated carbons.









Similar content being viewed by others
References
Adams, L.B., Hall, C.R., Holmes, R.J., Newton, R.A.: An examination of how exposure to humid air can result in changes in the adsorption properties of activated carbons. Carbon 26(4), 451–459 (1988)
Ahmad, F., Wan Daud, W.M.A.W., Ahmad, M.A., Radzi, R., Azmi, A.A.: The effects of CO2 activation, on porosity and surface functional groups of cocoa (Theobroma cacao): shell based activated carbon. J. Environ. Chem. Eng. 1, 378–388 (2013)
Ania, C.O., Parra, J.B., Pis, J.J.: Influence of oxygen-containing functional groups on active carbon adsorption of selected organic compounds. Fuel Process. Technol. 79, 265–271 (2002)
Azevedo, D.C.S., Araujo, J.C.S., Bastos-Neto, M., Torres, A.E.B., Jaguaribe, E.F., Cavalcante, C.L.: Microporous activated carbon prepared from coconut shells using chemical activation with zinc chloride. Microporous Mesoporous Mater. 100, 361–364 (2007)
Bandosz, T.J., Petit, C.: On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds. J. Colloid Interface Sci. 338, 329–345 (2009)
Barton, S.S., Evans, M.J.B., Liang, S., Macdonald, J.A.F.: The influence of surface modification of BPL carbons on aging. Carbon 34, 975–982 (1996)
Cecen, F., Aktas, O.: Activated carbon for water and wastewater treatment: integration of adsorption and biological treatment, 1st edn. Wiley, Weinhheim (2012)
Ehrburger, P., Henlin, J.M., Lahaye, J.: Aging of cupric oxide supported on activated carbon. J. Catal. 100, 429–436 (1986)
Ehrburger, P., Lahaye., J., Dziedzinl, P., Fangeat, R.: Effect of aging on the behavior of copper-chromium compounds supported on activated carbon. Carbon 29, 297–303 (1991)
Ghaedi, A.M., Ghaedi, M., Vafaei, A., Iravani, N., Keshavarz, M., Rad, M, Tyagi, I, Agarwal, S., Gupta, V.K.: Adsorption of copper(II) using modified activated carbon prepared from pomegranate wood: optimization by bee algorithm and response surface methodology. J. Mol. Liq. 206, 195–206 (2015)
Ghouma, I., Jeguirim, M., Dorge, S., Limousy, L., Ghimbeu, C., Ouederni, A.: Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. C. R. Chim. 18, 63–74 (2015)
Gupta, V.K., Gupta, B., Rastogi, A., Agarwal, S., Nayak, A.: A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-acid blue 113. J. Hazard. Mater. 186, 891–901 (2011)
Hall, C.R., Holmes, R.J.: The preparation and properties of some chlorinated activated carbons, part ii. further observations. Carbon 31, 881–886 (1993)
Hammarstrom, J.L., Sacco, A. Jr.: Investigation of deactivation mechanisms of ASC whetlerite charcoal. J. Catal. 112, 267–281 (1988)
Heidari, A., Younesi, H., Rashidi, A., Ghoreyshi, A.A.: Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem. Eng. J. 254, 503–513 (2014)
Karthikeyan, S., Sivakumar, P.: The effect of activating agents on the activated carbon prepared from feronia limonia (l.) swingle (wood apple) shell. J. Environ. Nanotechnol. 1, 05–12 (2012)
Kazemipour, M., Ansari, M., Tajrobehkar, S., Majdzadeh, M., Kermani, H.R.: Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond pistachio shell, and apricot stone. J. Hazard. Mater. 150(2), 322–327 (2008)
Kütahyalı, C., Eral, M.: Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J. Nucl. Mater. 396, 251–256 (2010)
Lodewyckx, P., Raymundo-Pinero, E., Vaclavikova, M., Berezovska, I., Thommes, M., Béguin, F., Dobos, G.: Suggested improvements in the parameters used for describing the low relative pressure region of the water vapour isotherms of activated carbons. Carbon 60, 556–558 (2013)
Ma, X., Ouyang, F.: Adsorption properties of biomass-based activated carbon prepared with spent coffee grounds and pomelo skin by phosphoric acid activation. Appl. Surf. Sci. 268, 566–570 (2013)
Mochizuki, T., Kubota, M., Matsuda, H., D’Elia Camacho, L.F.: Adsorption behaviors of ammonia and hydrogen sulfide on activated carbon prepared from petroleum coke by KOH chemical activation. Fuel Process. Technol. 144, 164–169 (2016)
Mohammad-khah, A., Ansari, M.: Activated charcoal: preparation, characterization and applications: a review article. Int. J. Chemtech. Res. 1(4), 859–864 (2009)
Moreno-Castilla, C., Carrasco-Marin, F., Maldonado-Hodar, F.J., Rivera-Utrilla, J.: Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content. Carbon 36, 145–151 (1998)
Nowicki, P., Wachowska, H., Pietrzak, R.: Active carbons prepared by chemical activation of plum stones and their application in removal of NO2. J. Hazard. Mater. 181, 1088–1094 (2010)
Nowicki, P., Kazmierczak, J., Pietrzak, R.: Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technol. 269, 312–319 (2015)
Petit, C., Bandosz, T.J.: Role of surface heterogeneity in the removal of ammonia from airon micro/mesoporous activated carbons modified with molybdenum and tungtsen oxides. Microporous Mesoporous Mater. 118, 61–67 (2009)
Rossin, J., Petersen, E., Tevault, D., Lamontange, R., Isaacson, L.: Effects of environmental weathering on the properties of asc whetlerite. Carbon 29, 197–205 (1991)
Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurin, G., Sing, K.: Adsorption by powders and porous solids. In: Principles, methodology and applications, 2nd ed. Academic Press, Oxford (2014)
Szymański, G.S., Karpinski, Z., Biniak, S., Swiatkowski, A.: The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon 40, 2627–2639 (2002)
Tazibet, S., Lodewyckx, P., Boucheffa, Y.: The influence of the cooling down step in the heat treatment on the stability of activated carbons hydrophobicity. Adsorption 20, 545–553 (2014)
Tazibet, S., Boucheffa, Y., Lodewyckx, P., Velasco, L.F., Boutillara, Y.: Evidence for the effect of the cooling down step on activated carbon adsorption properties. Microporous Mesoporous Mater. 221, 67–75 (2016)
Thommes, M.: Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 82, 1059–1073 (2010)
Thommes, M., Morell, J, Cychosz, K.A., Froba, M.: Combining nitrogen, argon, and water adsorption for advanced characterization of ordered mesoporous carbons (CMKs) and periodic mesoporous organosilicas (PMOs). Langmuir 29, 14893–14902 (2013)
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 87(9), 1051–1069 (2015)
Toth, A., Laszlo, K.: Water adsorption by carbons: hydrophobicity and hydrophilicity. Novel carbon adsorbents. Elsevier, Oxford (2012)
Velasco, L.F., Snoeck, D., Mignon, A., Misseeuw, L., Ania, C.O., Van Vlierberghe, S., Dubruel, P., De Belie, N., Lodewyckx, P.: Role of the surface chemistry of the adsorbent on the initialization step of the water sorption process. Carbon 106, 284–288 (2016)
Verhoeven, L., Lodewyckx, P.: Comparison of Dubinin-Radushkevich micropore volumes obtained from N2, CO2 and H2O adsorption isotherms. International Carbon Conference, Lexington (2001)
Vivo-Vilches, J.F., Bailón-García, E., Pérez-Cadenas, A.F., Carrasco-Marín, F., Maldonado-Hódar, F.J.: Tailoring activated carbons for the development of specific adsorbents of gasoline vapors. J. Hazard. Mater. 263, 533–540 (2013)
Yang, J.B., Ling, L.C., Liu, L., Kang, F.Y., Huang, Z.H., Wu, H.: Preparation and properties of phenolic resin-based activated carbon spheres with controlled pore size distribution. Carbon 40, 911–916 (2002)
Yorgun, S., Yildiz, D., Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 53, 122–131 (2015)
Zhao, S., Xiang, J., Wang, C.Y., Chen, M.M.: Characterization and electrochemical performance of activated carbon spheres prepared from potato starch by CO2 activation. J. Porous Mater. 20, 15–20 (2013)
Acknowledgements
The authors acknowledge the Department of Construction Engineering and Materials of the Royal Military Academy for the SEM and EDX analysis
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boutillara, Y., Velasco, L.F., Lodewyckx, P. et al. Textural and functional modifications of activated carbons subjected to severe storing conditions. Adsorption 24, 601–612 (2018). https://doi.org/10.1007/s10450-018-9979-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-018-9979-5