Abstract
The poly(vinyl alcohol) (PVA) influence on the adsorption and electrokinetic properties of the mixed oxide Mn x O y –SiO2/polymer solution system was examined. Three oxides differing with the Mn x O y contents were applied (0.2; 1 and 3 mmol/g SiO2, respectively). The PVA with the molecular weight 100 kDa was characterized with the acetate groups content equal to 14 %. Adsorption, solid surface charge and zeta potential measurements were made as a function of solution pH (3–10). The obtained results showed that the PVA adsorption amount strongly depends on not only the solution pH, but also manganese oxide content on the mixed oxide surface. The higher solution pH value (or Mn x O y content) is, the higher polymer adsorption is obtained. The PVA addition to the solid suspension causes minimal changes of the mixed oxide surface charge density, whereas the zeta potential of solid particles increases significantly in the polymer presence.
Similar content being viewed by others
1 Introduction
The application possibilities of metal oxides (including manganese oxides) in various branches of industry and ecology are large. For example MnO is commonly used as a component of fertilizers and food additives. On the other hand, MnO2 is a component of dry-cell and zinc–carbon batteries. Both solids also find application as inorganic pigments in ceramics and glassmaking. Their other usages include applications in catalysis (synthesis of allyl alcohols, CO oxidation), paints production, bleaching tallow and textile printing.
A very important aspect from the environmental point of view is the use of metal oxides obtained from removal of hazardous and undesirable substances from wastewaters. Recently manganese oxide coated zeolite was applied to remove Mn2+ from aqueous solutions (Taffarel and Rubio 2010). Manganese dioxide was also used as an adsorbent for phosphate in seawater (Yao and Millero 1996). Copper, cadmium, lead, zinc, iron, selenium and arsenic removal by the use of manganese oxide (both synthetic and natural minerals) was also examined (Kang et al. 2010; Puppa et al. 2013; Ergül et al. 2014; Demirkiran 2015).
However, metal oxides are often characterized by small specific surface area and inadequate stability of their aqueous suspension. In such a case, solid surface modification is necessary. Of great variety of techniques; plasma modification (Kuraica et al. 2003; Punga and Borcia 2013), adsorption of different low- and high-molecular compounds (Chibowski et al. 2010; Nosal-Wiercińska 2012, 2013; Wiśniewska et al. 2013a; Nosal-Wiercińska 2014) and synthesis of mixed oxides (Maliyekkal et al. 2006; Wu et al. 2010) acquired the most significant importance. Appropriate modification leads to obtaining solids of the properties desired in various ecological and technological processes (Kaźmierczak et al. 2013; Nowicki et al. 2014, 2015a, b).
Nanocomposites with nanosilica A-380 (Degussa) and grafted manganese oxide were prepared using manganese acetate—Mn(CH3COO)2·4H2O. The obtained aqueous dispersion was treated at 600 °C. Mn x O y –SiO2 mixed oxides (varying in Mn x O y content) obtained in such a way exhibit different surface characteristics and adsorption affinity for macromolecular compounds. They were also characterized by very large surface area. Different structure and surface properties of mixed oxides in comparison to simple oxides can change nature of interactions between the macromolecules and the solid surface. Thus, these substances can be used as effective adsorbents in many technological processes.
In the present study the mechanism of poly(vinyl alcohol) (PVA) adsorption on the surface of Mn x O y –SiO2 was investigated. This was done based on the results of measurements: spectrophotometric (determination of the adsorbed amount of the polymer on the solid surface), potentiometric titration (determination of the surface charge density of mixed oxide in the absence and presence of the PVA) and electrokinetic (zeta potential determination of Mn x O y –SiO2 particles covered and not with polymeric layers).
As follows from the obtained results the solution pH (in the range 3–10) affects the adsorption mechanism of PVA on the surface of mixed oxide. The polymer presence also has the effect on the structure of electrical double layers (edl) formed around solid particles.
The PVA was chosen for studies due to its extensive use in many branches of industry. PVA finds a wide application in the production of adhesives, coatings, medicines, paints, paper, oils, fibers and hydrogels (Hassan and Peppas 2000; Kadajji and Betageri 2011; Karimi and Navidbakhsh 2014). Polyvinyl alcohol is also used in food industry as an agent to retain satisfactory taste, texture and quality of the food (Gupta and Arora 2011).
The monomer of PVA–vinyl alcohol is very unstable and immediately undergoes isomerization to acetaldehyde. For this reason PVA is not prepared by polymerization of the corresponding monomer but in the polymerization process of vinyl acetate. Obtained polyvinyl acetate is converted to the PVA by the hydrolysis of its acetate groups to hydroxyl ones.
2 Experimental
2.1 Materials
Three samples of mixed oxides, consisting of silica (SiO2) and manganese oxide (Mn x O y ), were used as adsorbents. All solid samples were prepared in the Institute of Surface Chemistry of the National Academy of Sciences of Ukraine in Kiev. Mixed oxides were characterized by different Mn x O y contents, i.e. 0.2; 1 and 3 mmol/g SiO2 (they were designated as: 02 Mn–SiO2; 1 Mn–SiO2 and 3 Mn–SiO2, respectively). The characteristics of applied solids are given in Table 1. The solid BET surface area was obtained by the nitrogen adsorption–desorption method (Micrometritics ASAP 2405 analyzer).
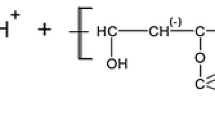
A PVA–PVA (Fluka) with a weight average molecular weight of 100 kDa, was applied as an adsorbate. The hydrolysis degree of PVA was 86 %. It means that 14 % of the acetate groups (–OCOCH3) do not hydrolyze to hydroxyl (–OH) ones in its production process. PVA is classified as a nonionic polymer, however, its macromolecules comprise a number of acetate groups (as a result of incomplete hydrolysis). These groups undergo ionization with the increasing solution pH, as was schematically shown below.

Therefore the applied polymer has negative charges which can interact electrostatically with oppositely charged groups on the solid surface.
All measurements were performed at 25 °C in the pH range 3–10, using a solution of NaCl with the concentration of 0.01 mol/dm3 as the supporting electrolyte.
2.2 Methods
The amount of PVA adsorbed on the surface of the mixed oxides was determined by the static method. The decrease in polymer concentration in the solution after the adsorption process was detected. For this purpose the PVA reaction with H3BO3 and I2 solutions (Zwick 1965) were applied. As a result, the green colour of the PVA solution of varying intensity (depending on the polymer concentration) was obtained. Absorbancies of these solutions were measured using a UV/VIS spectrophotometer Cary 100 (Varian).
First, three series of PVA solutions were prepared (seven solutions in each). The polymer concentrations for each series were: 30, 50, 70, 100, 200, 300 and 400 ppm. Then the appropriate pH (3, 6, 9 ± 0.1) was adjusted. The pH values of the examined system were adjusted using a pH-meter PHM 240 (Radiometer) with the accuracy ±0.1.
After 15 min from the addition of appropriate reagents (causing green colour of the solution), absorbancies were measured at the wavelength 682 nm. These measurements were performed using quartz cuvettes. Based on these results calibration curves, showing the absorbance versus the concentration of the polymer solution, were prepared.
The PVA concentration in the solution was determined in an analogous way. To the polymer solutions of the concentrations ranging from 30 to 400 ppm, 0.005 g of the solid was added and the appropriate pH was adjusted. The adsorption process was carried out under the conditions of continuous shaking for approx. 24 h [using a shaker Unimax 1010 (Heidolph) with the attached heating module (type Incubator 1000)]. It should be noted that adsorption equilibrium in examined systems was achieved after about 6 h. Then, the samples were centrifuged [centrifuge type 223e (MPW Med. Instruments)] and the concentration of PVA in the solution was determined (using respective calibration curves). From the difference of polymer concentration before and after adsorption, the PVA amount adsorbed on the mixed oxide surface was determined.
For the potentiometric titration, a set consisting of: thermostated Teflon vessel, glass and calomel electrodes (Beckman Instruments), pH-meter PHM 240 (Radiometer), laboratory mixers, thermostat RE 204 (Lauda), automatic microburette Dosimat 765 (Metrohm) and computer, was applied. Titrations were performed using the software “titr_v3” authored by prof. W. Janusz. This program enables solid surface charge density calculation and oxide pHpzc (point of zero charge) determination.
Potentiometric titration started with the preparation of 50 cm3 of supporting electrolyte in the thermostatted Teflon vessel. To obtain pH in the range 3–3.5, 0.2 cm3 HCl (with the concentration 0.1 mol/dm3) was added. After equilibrium was reached, the examined system was titrated with a NaOH solution (with the concentration 0.1 mol/dm3). As a result the dependence of the titrated solution pH as a function of added base volume (reference or electrolyte curve) was obtained. Identical potentiometric titration was performed in the systems: Mn x O y –SiO2/electrolyte solution and Mn x O y –SiO2/PVA solution. The masses of applied solids were: 0.065 g for 02 Mn–SiO2; 0.076 g for 1 Mn–SiO2 and 0.107 g for 3 Mn–SiO2. The polymer concentration in the system was 100 ppm.
The zeta potential of mixed oxides particles (without and with PVA adsorption layers) was measured using a Zetasizer Nano ZS (Malvern Instruments). Suspensions were prepared by adding 0.01 g of the solid to 50 cm3 of the electrolyte or polymer solution (100 ppm). After the suspension sonication for 5 min [ultrasonicator XL 2020 (Misonix)], the solution was poured into seven 10 cm3 Erlenmayer flasks. In each of them the appropriate pH value was adjusted (i.e. 3; 4; 5; 6; 7; 8; 9, respectively). The ζ potential of such prepared suspension was measured using the dip cell. Before each experiment the cell was rinsed twice with the suspension under investigation. Zeta potential was calculated using the special computer program making conversion of the electrophoretic mobility of particles in the zeta potential. For this purpose the Smoluchowski equation was applied. The final value of the electrokinetic potential was the average of five measurements.
3 Results and Discussion
Figure 1 presents the adsorption isotherms of PVA on the surface of mixed oxide 1 Mn–SiO2 for different solution pH. As can be seen, PVA adsorption increases with the pH rise. The similar dependencies were obtained for two other examined oxides (i.e. 02 Mn–SiO2 and 3 Mn–SiO2). On the other hand, Fig. 2 shows the adsorbed amounts of PVA on the surface of mixed oxide for different contents of Mn. The increase in the manganese oxide content on the mixed oxide surface results in higher adsorption of polyalcohol.
Such adsorption behaviour of the polymer is related to the conformation of its macromolecules under specified pH conditions. As mentioned earlier, the chains of PVA contain a certain number of acetate groups (14 %), which are the source of the negative charge of the polymer molecules. Even such a relatively small part of these groups affects the structure of the PVA adsorption layer. Our previous study indicated that the acetate groups play an essential role in the PVA adsorption—the increase of the number of these groups in the polymeric chains led to higher adsorption levels (Chibowski et al. 2000). Contribution of charged acetate groups rises as the solution pH increases. It results in larger extension of polymer chains due to the electrostatic repulsion of negative charges located along polymeric chains. The degree of the polymer macromolecules development directly affects the amount of PVA adsorbed on the Mn x O y –SiO2 surface.
At pH 3 macromolecular compound chains adopt the least developed conformation. They form loop and tail structures of small length on the solid surface. Such flat arrangement of the adsorbed macromolecule results in the occupation of large surface area of the mixed oxide. This leads to the blockade of other PVA chains access to the solid active sites. It is worth noting that under these conditions mixed oxide is positively charged (pHpzc in the range 6.88–8.01, Table 1). Thus, there is electrostatic attraction between the Mn x O y –SiO2 surface and the PVA macromolecules (slightly negatively charged). This phenomenon contributes also to the adoption of flatter structure of the polymer chains in surface layer. Such adsorption mechanism causes obtaining the lowest level of PVA adsorption at the smallest examined pH value.
Intermediate values of the PVA adsorbed amounts were obtained at pH 6. This is due to a larger content of charged segments with the acetate groups which mutually repeal each other. As a result, the polymer macromolecules assume more stretched conformation than in the solution of pH 3. The structure of the polymeric adsorption layer formed on the solid surface is characterized by a greater length of the loop and tail fragments of PVA chains. The adsorbed PVA macromolecules occupy a smaller surface area than that at pH 3. This allows greater packing density of the polymeric layer to be formed at the solid–liquid interface, resulting in the observed adsorption increase. Additionally, at pH 6 the electrostatic adsorbent–adsorbate attraction is weaker than at pH 3 (lower positive charge of the mixed oxide surface). This also favours formation of the thicker layers by PVA chains on the solid surface.
At pH 9, practically all the acetate groups of the polymer macromolecules are endowed with a negative charge. As a result, electrostatic repulsion of charged segments is the strongest. These interactions lead to the greatest development of the polyalcohol chains. On the other hand, the solid surface is also negatively charged. This additionally intensifies the degree of PVA macromolecules stretching. Polymeric molecules possessing such a conformation can be adsorbed in large amounts on the solid surface. At pH 9 polymer tails and loops are the longest and PVA adsorption is the highest.
The obtained results proved that adsorbent–adsorbate electrostatic repulsion does not prevent adsorption of PVA on the surface of the mixed oxide. On the contrary, it promotes the formation of a specific conformation of the adsorbed macromolecule that provides the highest level of adsorption. Under such unfavourable electrostatic conditions, PVA segments are directly bounded with the solid surface active sites through hydrogen bonds (Kasprzyk-Hordern 2004). These bonds can be formed between all types of surface groups (charged: –MO−, –MOH2 + and uncharged –MOH, where M is atom of Si or Mn) and functional groups of polymer—hydroxyl and acetate (both neutral and ionized).
It was demonstrated that the PVA adsorption on the surface of the mixed oxide depends strongly on the manganese oxide content in the solid structure (Fig. 2). The smallest PVA adsorbed amount is observed on the surface of the mixed oxide 02 Mn–SiO2 and the highest—for 3 Mn–SiO2. For example at pH 9 over twice higher PVA adsorption occurs on the 3 Mn–SiO2 surface in comparison to that on the 02 Mn–SiO2 surface. The analogous tendency is observed for all examined solution pH values.
The increase in PVA adsorption with the increase of manganese content on the surface of the mixed oxide proves that solid active sites containing Mn atoms are mainly responsible for polymer binding. The role of active sites containing Si atoms in this process must be considerably smaller. In aqueous solution metal atoms undergo hydroxylation. Formed hydroxyl groups are involved in hydrogen bond formation between the Mn x O y –SiO2 surface and poly(vinyl alcohol macromolecules). Moreover, hydroxyl groups on the solid surface are amphoteric and can connect or disconnect proton. This leads to the formation of electrical charge which can interact with the ionized groups of PVA. A higher content of metal in the structure of the mixed oxide is equivalent to a greater number of possible interactions with the adsorbate molecules. As a consequence, higher PVA adsorption occurs on the surface of the mixed oxide Mn x O y –SiO2 characterized by the greatest Mn content.
Figures 3 and 4 present the results of potentiometric titrations in the mixed oxide/solution systems as dependencies of the solid surface charge density (σ0) versus solution pH. The influence of manganese oxide content on the surface charge density of Mn x O y –SiO2 mixed oxides without polymer is shown in Fig. 3. As can be seen, the point of zero charge of the Mn x O y –SiO2 surface is in the range of 6.88–8.01. The higher content of Mn in the surface structure of the solid indicated that the greater value of pHpzc is reached. This is probably caused by the formation of a greater number of positively charged –MnOH2 + surface groups. Evidence of this is also increase in positive value of σ0 in the pH range 4–8 with the rise of manganese oxide content. It is worth noting that the addition of manganese on the silica surface leads to modification of the solid characteristics. This manifests in a noticeable shift of pHpzc position (towards the more alkaline pH) in relation to that of SiO2 whose value is about 3 (Wiśniewska 2012a, b, 2013b).
The analysis of the dependencies in Fig. 4 leads to the conclusion that adsorption of PVA on the 1 Mn–SiO2 surface practically does not change the surface charge of the solid. The similar data are obtained for the other examined systems. Such behaviour occurs in the case of nonionic polymers and confirms that hydrogen bonds are responsible for the PVA chains adsorption on the Mn x O y –SiO2 surface. Although PVA contains ionizable acetate groups, their content of in the PVA macromolecules (14 %) is insufficient to cause noticeable changes in σ0 of the adsorbent. It should be also added that negative charge of poly(vinyl alcohol chains) is due to the existence of resonant structure of polymer macromolecule and migration of partial negative charge from the –CH2 − groups located at α positions relative to the acetate groups to carbonyl oxygen of the acetate group (and conversely). This dispersion of negative charge on various chain fragments makes its influence on the solid surface charge density minimal.
Next to the solid surface charge, the electrokinetic potential (ζ) is an equally important parameter characterizing the structure of the edl formed at the mixed oxide/solution interface. The results of zeta potential measurements are given in Figs. 5, 6, and 7. The analysis of dependencies obtained for the mixed oxides in the supporting electrolyte solution (without PVA) indicates that ζ potential is negative in the whole range of studied pH and its absolute values increase with the pH rise. This means that in the slipping plane within edl, negative ions of supporting electrolyte (Cl−) predominate. Very interesting is the influence of manganese oxide content on the zeta potential of Mn x O y –SiO2 mixed oxides particles without polymer (Fig. 5). The smallest absolute values of electrokinetic potential are obtained for the 1 Mn–SiO2 system, whereas the greatest ones for the 3 Mn–SiO2 system. The zeta potentials characterizing 02 Mn–SiO2 (with the smallest content of manganese oxide) assume intermediate values. The highest Mn content in the solid structure (and thus a larger content of –MnOH2 + surface groups) requires attachment of a greater number of supporting electrolyte counter-ions in the Stern layer of edl.
The addition of PVA causes a decrease in the absolute value of the electrokinetic potential, whereas its sign remains negative. This decrease is the smallest for the 02 Mn–SiO2 system (Fig. 6) and the greatest for the 3 Mn–SiO2 system (Fig. 7). Three different effects can be responsible for the changes in the zeta potential in the presence of polymer. There are: (1) the shift of the slipping plane from the solid surface due to polymeric adsorption layer formation, (2) the presence of the charges coming from the PVA acetate groups in the by-surface layer of the solution, (3) the displacement of the counter-ions from the slipping plane layer caused by the polymer binding with the oxide surface (Vincent 1974). In the summary, these effects overlap and give final values of zeta potential of the solid suspensions containing PVA. The main effect in the examined Mn x O y –SiO2/NaCl/PVA systems seems to be displacement of the supporting electrolyte ions by the loop and tail structures of the adsorbed polymer chains from the surface layer to the slipping plane. It changes composition of the electrical double layer in relation to simple electrolyte ions. On the other hand, the presence of negatively charged acetate groups in the polymeric chains and the slipping plane shift due to the polymer adsorption can also affect the zeta potential of the examined systems.
4 Conclusions
The amount of PVA adsorbed on the Mn x O y –SiO2 surface depends on the solution pH, the content of manganese oxide in the structure of the solid and the ionization degree of the acetate groups in the polymer macromolecules. The specific conformation of the adsorbed PVA macromolecules assumed under given pH conditions is responsible for PVA adsorption increase with the pH rise. At pH 3 the adsorbing PVA macromolecules are more coiled (low ionization of acetate groups in the polymeric chains) and form rather flat structure on the solid surface (slight solid-PVA electrostatic attraction). This leads to the blockade of solid active sites by polymeric coils and the lowest PVA adsorption level. On the contrary, at pH 9 a thicker adsorption layer is formed due to solid-polymer repulsion and a greater extent of adsorbing macromolecules (total ionization of their acetate groups). As a result, greater packing of the adsorbed PVA chains on the surface unit is possible leading to the increase of PVA adsorption. The higher is the manganese content on the mixed oxide surface, the larger is the amount of adsorbed polymer. It is related to a greater number of possible connections between the polymer segments and the solid surface group containing Mn atoms.
The modification of silica surface with manganese oxide results in change of its properties. The value of their pHpzc points increases from 6.88 to 8.01 with the increasing Mn content. Adsorption of PVA minimally affects the surface charge density of mixed oxides (hydrogen bridges are mainly responsible for PVA adsorption). The zeta potential of mixed oxide particles is negative in the whole range of studied pH. The PVA addition results in significant increase of the zeta potential values in all examined Mn x O y –SiO2 systems in relation to the suspensions without polymer.
References
Chibowski, S., Paszkiewicz, M., Krupa, M.: Investigation of the influence of the polyvinyl alcohol adsorption on the electrical properties of Al2O3–solution interface, thickness of the adsorption layers of PVA. Powder Tech. 107, 251–255 (2000)
Chibowski, S., Wiśniewska, M., Urban, T.: Influence of solution pH on stability of aluminum oxide suspension in presence of polyacrylic acid. Adsorption 16, 321–332 (2010)
Demirkiran, N.: Copper adsorption by natural manganese dioxide. Trans. Nonferr. Met. Soc. China 25, 647–653 (2015)
Ergül, B., Bektas, N., Öncel, M.S.: The use of manganese oxide minerals for the removal arsenic and selenium anions from aqueous solutions. Energy Environ. Eng. 2, 103–112 (2014)
Gupta, A.P., Arora, G.: Preparation and characterization of guar-gum/polyvinylalcohol blend films. J. Mat. Sci. Eng. B 1, 28–33 (2011)
Hassan, C.M., Peppas, N.A.: Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 153, 37–65 (2000)
Kadajji, V.G., Betageri, G.V.: Water soluble polymers for pharmaceutical applications. Polymers 3, 1972–2009 (2011)
Kang, D.H., Schwab, A.P., Johnston, C.T., Banks, M.K.: Adsorption of iron cyanide complexes onto clay minerals, manganese oxide, and soil. J. Environ. Sci. Health. A 45, 1391–1396 (2010)
Karimi, A., Navidbakhsh, M.: Mechanical properties of PVA material for tissue engineering applications. Mater. Technol. 29, 90–100 (2014)
Kasprzyk-Hordern, B.: Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 110, 19–48 (2004)
Kaźmierczak, J., Nowicki, P., Pietrzak, R.: Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 19, 273–281 (2013)
Kuraica, M.M., Astashynski, V.M., Dojcinovic, I.P., Puric, J.: Modification of solid surface by a compression plasma flow. Phys. Laser Cryst. 126, 245–255 (2003)
Maliyekkal, S.M., Sharma, A.K., Philip, L.: Manganese-oxide-coated alumina: a promising sorbent for defluoridation of water. Water Res. 40, 3497–3506 (2006)
Nosal-Wiercińska A.: Intermolecular interactions in systems containing Bi(III)–\(\text{ClO}_{4}^{ - }\)–H2O–selected amino acids in the aspect of catalysis of Bi(III) electroreduction. Electroanalysis 26, 1013–1023 (2014)
Nosal-Wiercińska, A.: The role of active complexes in the multistep process of Bi(III) ion electroreduction in chlorate (VII) solutions with varied water activity in the presence of cystine. Electrochim. Acta 92, 397–403 (2013)
Nosal-Wiercińska, A.: Electrochemical and thermodynamic study of the electroreduction of Bi(III) ions in the presence of cystine in solutions of different water activity. J. Electroanal. Chem. 681, 103–108 (2012)
Nowicki, P., Skibiszewska, P., Pietrzak, R.: Hydrogen sulphide removal on carbonaceous adsorbents prepared from coffee industry waste materials. Chem. Eng. J. 248, 208–215 (2014)
Nowicki, P., Kaźmierczak, J., Pietrzak, R.: Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technol. 269, 312–319 (2015a)
Nowicki, P., Kaźmierczak, J., Sawicka, K., Pietrzak, R.: Nitrogen-enriched activated carbons prepared by the activation of coniferous tree sawdust and their application in the removal of nitrogen dioxide. Int. J. Environ. Sci. Technol. 12, 2233–2244 (2015b)
Punga, I.L., Borcia, G.: Surface modification and stability of polymers treated by atmospheric-pressure, helium plasma. J. Adv. Res. Phys. 4, 011305 (2013)
Puppa, L.D., Komarek, M., Bordas, F., Bollinger, J.C., Joussein, E.: Adsorption of copper, cadmium, lead and zinc onto a synthetic manganese oxide. J. Colloid Interface Sci. 399, 99–106 (2013)
Taffarel, S.R., Rubio, J.: Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Min. Eng. 23, 1131–1138 (2010)
Vincent, B.: The effect of adsorbed polymers on dispersion stability. Adv. Colloid Interface Sci. 4, 193–277 (1974)
Wiśniewska, M., Nosal Wiercińska, A., Dąbrowska, I., Szewczuk-Karpisz, K.: Effect of the solid pore size on the structure of polymer film at themetal oxide/polyacrylic acid solution interface – temperaturę impact. Microporous Mesoporous Mater. 175, 92–98 (2013a)
Wiśniewska, M., Szewczuk-Karpisz, K., Ostolska, I.: Temperature effect on the adsorption equilibrium at the silica - polyethylene glycol solution interface. Fluid Ph. Equilb. 360, 10–15 (2013b)
Wiśniewska, M.: Temperature effects on the adsorption polyvinyl alcohol on silica. Cent. Eur. J. Chem. 10, 1236–1244 (2012a)
Wiśniewska, M.: The temperature effect on the adsorption mechanism of polyacrylamide on the silica surface and its stability. Appl. Surf. Sci. 258, 3094–3101 (2012b)
Wu, X., Lin, F., Xu, H., Weng, D.: Effects of adsorbed and gaseous NO x species on catalytic oxidation of diesel soot with MnOx–CeO2 mixed oxides. Appl. Cat. B. 96, 101–109 (2010)
Yao, W., Millero, F.J.: Adsorption of phosphate on manganese dioxide in seawater. Environ. Sci. Technol. 30, 536–541 (1996)
Zwick, M.M.: Poly(vinyl alcohol)-iodine complexes. J. Appl. Polym. Sci. 9, 2393–2424 (1965)
Acknowledgments
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/under REA Grant agreement n° PIRSES-GA-2013-612484.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wiśniewska, M., Bogatyrov, V., Ostolska, I. et al. Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide Mn x O y –SiO2 . Adsorption 22, 417–423 (2016). https://doi.org/10.1007/s10450-015-9696-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9696-2