Abstract
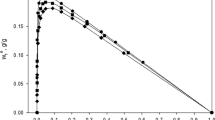
Application of the MPTA model has been extended to associative liquid adsorption. The MPTA model describes fluid–fluid interactions using an equation of state (EoS) term, and fluid–solid interactions using a potential equation. In order to extend the application to associative liquid adsorption, an association term has been considered for fluid–fluid interactions. Sixteen binary mixtures containing associating and non-associating components in equilibrium with various adsorbents have been studied; fluid–fluid interactions have been modeled using the Peng–Robinson, Soave–Redlich–Kwong, volume-translated SRK and CPA equations of state, while the effects of fluid–solid interactions have been taken into account using Dubinin–Radushkevich–Astakhov (DRA) and Steele potential functions. The model parameters have been obtained by fitting the model to experimental data on surface excess. For the studied systems, the accuracy of fitted isotherms has been found to be more dependent on the fluid–solid potential equation rather than the applied EoS. Calculations show that the SRK equation is a suitable choice for non-associating systems, while the CPA equation is found to be more appropriate for associating systems, as would be expected. The results also show that the Steele potential function is in better agreement with experimental data than the DRA potential function.







Similar content being viewed by others
Abbreviations
- AARD :
-
Average absolute relative deviation, \(\frac{100}{N}\sum {\left| {\frac{{n_{i}^{e,\exp } - n_{i}^{e,calc} }}{{n_{i}^{e,\exp } }}} \right|}\)
- N :
-
Number of experimental data
- P :
-
Pressure (bar)
- P(z):
-
Pressure at given z value (bar)
- x B :
-
Mole fraction of bulk phase
- x(z):
-
Mole fraction of adsorbed phase at given z value
- z :
-
Distance (cm) or pore volume (cm3/gads)
- ε i :
-
Adsorption potential of “i” specie (J/mol)
- φ i :
-
Fugacity coefficient of “i” specie
- μ i :
-
Chemical potential of “i” specie (J/mol)
- ρ(z):
-
Density of adsorbed phase at given z value
- ρ B :
-
Density of bulk phase
- \(\varGamma_{i}^{(n)}\) :
-
Surface excess of “i” specie (mol/gads)
- AARD:
-
Absolute average relative deviation
- ASST:
-
Adsorbate solid solution theory
- CPA:
-
Cubic plus association
- DRA:
-
Dubinin–Radushkevich–Astakhov
- EoS:
-
Equation of state
- IAST:
-
Ideal adsorbed solution theory
- MPTA:
-
Multicomponent potential theory of adsorption
- PAH:
-
Polycyclic aromatic hydrocarbon
- PR:
-
Peng–Robinson
- PTA:
-
Potential theory of adsorption
- RAST:
-
Real adsorbed solution theory
- SRK:
-
Soave–Redlich–Kwong
- VOC:
-
Volatile organic compound
- VST:
-
Vacancy solution theory
- VT-SRK:
-
Volume translated Soave–Redlich–Kwong
References
Balilehvand, S., Hashemianzadeh, S.M., Razavi, S.S., Karimi, H.: Investigation of hydrogen and methane adsorption/separation on silicon nanotubes: a hierarchial multiscale method from quantum mechanics to molecular simulation. Adsorption 18, 13–22 (2012)
Bjørner, M.G., Shapiro, A.A., Kontogeorgis, G.M.: Potential theory of adsorption for associating mixtures: possibilities and limitations. Ind. Eng. Chem. Res. (2013). doi:10.1021/ie302144t
Berti, C., Ulpig, P., Schulz, A.: Correlation and prediction of adsorption from liquid mixtures on solids by use of GE-models. Adsorption 6, 79–91 (2000)
Chae, K., Violi, A.: Mutual diffusion coefficients of heptane isomers in nitrogen: a molecular dynamics study. Chem. Phys. 134, 044537 (2011)
Daifullah, A.A.M., Girgis, B.S.: Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A 214(1–3), 181–193 (2003). doi:10.1016/S0927-7757(02)00392-8
Duan, Q.Y., Gupta, V.K., Sorooshian, S.: Shuffled complex evolution approach for effective and efficient global minimization. Optim. Theory Appl. 76(3), 501–521 (1993)
Dubinin, M., Astakhov, V.: Description of adsorption equilibria of vapors on zeolites over wide ranges of temperature and pressure. Adv. Chem. Ser. 102, 69–85 (1971)
Frahn, J., Malsch, G., Matuschewski, H., Schedler, U., Schwarz, H.-H.: Separation of aromatic/aliphatic hydrocarbons by photo-modified poly(acrylonitrile) membranes. J. Membr. Sci. 234(1–2), 55–65 (2004). doi:10.1016/j.memsci.2003.12.017
Fukuchi, K.: Application of vacancy solution theory to adsorption from dilute aqueous solutions. J. Chem. Eng. Jpn. 15, 316 (1982)
Goworek, J., Nieradka, A.: Adsorption from ternary liquid mixtures on partially dehydroxylated silica gel. J. Colloid Interface Sci. 180(2), 371–376 (1996). doi:10.1006/jcis.1996.0315
Kalies, G., Bräuer, P., Rouquerol, F.: Prediction of ternary liquid adsorption on solids from binary data. J. Colloid Interface Sci. 229(2), 407–417 (2000). doi:10.1006/jcis.2000.7036
Kalies, G., Rockmann, R., Tuma, D., Gapke, J.: Ordered mesoporous solids as model substances for liquid adsorption. Appl. Surf. Sci. 256(17), 5395–5398 (2010). doi:10.1016/j.apsusc.2009.12.091
Khan, A.R., Riazi, M.R., Al-Roomi, Y.A.: A thermodynamic model for liquid adsorption isotherms. Sep. Purif. Technol. 18(3), 237–250 (2000). doi:10.1016/S1383-5866(00)00052-6
Khanal, O.R.: Organics in atmospheric particulates. Ph.D thesis, The University of Auckland (2003)
Kontogeorgis, G.M., Yakoumis, I.V., Meijer, H., Hendriks, E., Moorwood, T.: Multicomponent phase equilibrium calculations for water–methanol–alkane mixtures. Fluid Phase Equilib. 158–160, 201–209 (1999). doi:10.1016/S0378-3812(99)00060-6
Lee, C.S., Belfort, G.: Thermodynamics of multiorganic solute adsorption from dilute aqueous solution. 1. The use of an equation of state. Ind. Eng. Chem. Res. 27(6), 951–956 (1988). doi:10.1021/ie00078a010
Lin, S.H., Huang, C.Y.: Adsorption of BTEX from aqueous solutions by macroreticular resins. Hazard. Mater. 70, 21–37 (1999)
Lin, H., Duan, Y.-Y., Zhang, T., Huang, Z.-M.: Volumetric property improvement for the Soave–Redlich–Kwong equation of state. Ind. Eng. Chem. Res. 45(5), 1829–1839 (2006). doi:10.1021/ie051058v
Long, C., Li, Q., Li, Y., Liu, Y., Li, A., Zhang, Q.: Adsorption characteristics of benzene–chlorobenzene vapor on hypercrosslinked polystyrene adsorbent and a pilot-scale application study. Chem. Eng. J. 160(2), 723–728 (2010). doi:10.1016/j.cej.2010.03.074
Monsalvo, M.A., Shapiro, A.A.: Modeling adsorption of binary and ternary mixtures on microporous media. Fluid Phase Equilib. 254(1–2), 91–100 (2007a). doi:10.1016/j.fluid.2007.02.006
Monsalvo, M.A., Shapiro, A.A.: Prediction of adsorption from liquid mixtures in microporous media by the potential theory. Fluid Phase Equilib. 261(1–2), 292–299 (2007b). doi:10.1016/j.fluid.2007.07.067
Monsalvo, M.A., Shapiro, A.A.: Modeling adsorption of liquid mixtures on porous materials. J. Colloid Interface Sci. 333(1), 310–316 (2009a). doi:10.1016/j.jcis.2009.01.055
Monsalvo, M.A., Shapiro, A.A.: Study of high-pressure adsorption from supercritical fluids by the potential theory. Fluid Phase Equilib. 283(1–2), 56–64 (2009b). doi:10.1016/j.fluid.2009.05.015
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11(1), 121–127 (1965). doi:10.1002/aic.690110125
Nevin Yalcin, V.S.: Study of surface area and porosity of activated carbon prepared from rice husk. Carbon 38, 1943–1945 (2000)
Peng, D.-Y., Robinson, D.B.: A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15(1), 59–64 (1976). doi:10.1021/i160057a011
Poiling, B.E., Prausnitz, J.M., O’Connell, J.P.: The Properties of Gases and Liquids, 5th edn. McGraw-Hill, New York (2000)
Rockmann, R., Kalies, G.: Liquid adsorption of n-octane/octanol/ethanol on SBA-16 silica. J. Colloid Interface Sci. 315(1), 1–7 (2007). doi:10.1016/j.jcis.2007.06.029
Ruthven, D.M.: Principles of adsorption and adsorption processes. Wiley, New York (1976)
Sene, L., Converti, A., Felipe, M.G.A., Zilli, M.: Sugarcane bagasse as alternative packing material for biofiltration of benzene polluted gaseous streams: a preliminary study. Bioresour. Technol. 83, 153–157 (2002)
Shapiro, A.A., Stenby, E.H.: Potential theory of multicomponent adsorption. J. Colloid Interface Sci. 201(2), 146–157 (1998). doi:10.1006/jcis.1998.5424
Soares, A., Albergaria, J.T., Domingues, V.F., Alvim-Ferraz, M.D.M., Delerue-Matos, C.: Remediation of soils combining soil vapor extraction and bioremediation: benzene. Chemosphere 80, 823–828 (2010)
Soave, G.: Equilibrium constants from a modified Redlich–Kwong equation of state. Chem. Eng. Sci. 27(6), 1197–1203 (1972). doi:10.1016/0009-2509(72)80096-4
Steele, W.A.: The interaction of gases with solid surfaces. Pergamon, Oxford (1974)
Stoeckli, F.: Recent developments in Dubinin’s theory. Carbon 36(4), 363–368 (1998). doi:10.1016/S0008-6223(97)00194-2
Suzuki, M.: Adsorption engineering. Kodansha (1990)
Świątkowski, A., Deryło-Marczewska, A., Goworek, J., Błażewicz, S.: Study of adsorption from binary liquid mixtures on thermally treated activated carbon. Appl. Surf. Sci. 236(1–4), 313–320 (2004). doi:10.1016/j.apsusc.2004.05.003
Tham, Y.J., Latif, P.A., Abdullah, A.M., Shamala-Devi, A., Taufiq-Yap, Y.H.: Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 102, 724–728 (2011)
Wells, A.F., Wells, A.: Structural inorganic chemistry, vol. 707. Clarendon Press, Oxford (1975)
Yin, C.Y., Aroua, M.K., Daud, W.M.A.W.: Review of modification of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 52, 403–415 (2007)
Acknowledgments
The authors appreciate precious assistance from Professor Alexander A. Shapiro and wish to acknowledge his hints and co-operation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naseri, A.A., Dehghani, M.R. & Behzadi, B. Modeling adsorption in binary associating solvents using the extended MPTA model. Adsorption 20, 555–563 (2014). https://doi.org/10.1007/s10450-013-9600-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9600-x