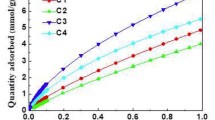
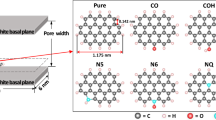
Abstract
A combined experimental and molecular simulation study of the coadsorption of CO2 and CH4 in porous carbons is reported. We address the effect of surface chemistry by considering a numerical model of disordered porous carbons which has been modified to include heterochemistry (with a chemical composition consistent with that of the experimental sample). We discuss how realistic the numerical sample is by comparing its pore size distribution (PSD), specific surface area, porous volume, and porosity with those for the experimental sample. We also discuss the different criteria used to estimate the latter properties from a geometrical analysis. We demonstrate the ability of the MP method to estimate PSD of porous carbons from nitrogen adsorption isotherms. Both the experimental and simulated coadsorption isotherms resemble those obtained for pure gases (type I in the IUPAC classification). On the other hand, only the porous carbon including the heterogroups allows simulating quantitatively the selectivity of the experimental adsorbent for different carbon dioxide/methane mixtures. This result shows that taking into account the heterochemistry present in porous carbons is crucial to represent correctly adsorption selectivities in such hydrophobic samples. We also show that the adsorbed solution theory describes quantitatively the simulated and experimental coadsorption isotherms without any parameter adjustment.







Similar content being viewed by others
References
Aaron, D., Tsouris, C.: Separation of CO2 from flue gas: a Review. Sep. Sci. Technol 40, 321–348 (2005)
Bae, J.S., Bhatia, S.K.: High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 20, 2599–2607 (2006)
Bhattacharya, S., Coasne, B., Hung, F.R., Gubbins, K.E.: Modeling micelle-templated mesoporous material SBA-15: atomistic model and gas adsorption studies. Langmuir 25, 5802–5813 (2009)
Bhattacharya, S., Gubbins, K.E.: Fast method for computing pore size distributions of model materials. Langmuir 22, 7726–7731 (2006)
Billemont, P., Coasne, B., De Weireld, G.: An experimental and molecular simulation study of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water. Langmuir 27, 1015–1024 (2011)
Billemont, P., Coasne, B., De Weireld, G.: Adsorption of carbon dioxide, methane, and their mixtures in porous carbons: effect of surface chemistry, water content, and pore disorder. Langmuir 29, 3328–3338 (2013)
Brochard, L., Vandamme, M., Pellenq, R.J.M.: Poromechanics of microporous media. J. Mech. Phys. Solids 60, 606–622 (2012a)
Brochard, L., Vandamme, M., Pellenq, R.J.M., Fen-Chong, T.: Adsorption-induced deformation of microporous materials: coal swelling induced by co2–ch4 competitive adsorption. Langmuir 28, 2659–2670 (2012b)
Busch, A., Gensterblum, Y., Krooss, B.M., Littke, R.: Methane and carbon dioxide adsorption–diffusion experiments on coal: upscaling and modeling. Int. J. Coal Geol 60, 151–168 (2004)
Chen, G., Yang, J., Liu, Z.: Method for simultaneous measure of sorption and swelling of the block coal under high gas pressure. Energy Fuels 26, 4583–4589 (2012)
Clauzier, S., Ho, L.N., Pera-Titus, M., Coasne, B., Farrusseng, D.: Enhanced H2 uptake in solvents confined in mesoporous metal–organic framework. J. Am. Chem. Soc 134, 17369–17371 (2012)
Coasne, B., Alba-Simionesco, C., Audonnet, F., Dosseh, G., Gubbins, K.E.: Adsorption and structure of benzene on silica surfaces and in nanopores. Langmuir 25, 10648–10659 (2009)
Coasne, B., Alba-Simionesco, C., Audonnet, F., Dosseh, G., Gubbins, K.E.: Adsorption, structure and dynamics of benzene in ordered and disordered porous carbons. Phys. Chem. Chem. Phys 13, 3748–3757 (2011)
Coasne, B., Czwartos, J., Gubbins, K.E., Hung, F.R., Sliwinska-Bartkowiak, M.: Freezing and melting of binary mixtures confined in a nanopore. Mol. Phys 102, 2149–2163 (2004)
Coasne, B., Czwartos, J., Gubbins, K.E., Hung, F.R., Sliwinska-Bartkowiak, M.: Freezing of mixtures confined in a slit nanopore. Adsorption 11, 301–306 (2005)
Coasne, B., Jain, S., Gubbins, K.: Adsorption, structure and dynamics of fluids in ordered and disordered models of porous carbons. Mol. Phys 104, 3491–3499 (2006)
Coasne, B., Jain, S.K., Naamar, L., Gubbins, K.E.: Freezing of argon in ordered and disordered porous carbon. Phys. Rev. B 76, 085416 (2007)
Coasne, B., Ugliengo, P.: Atomistic model of micelle-templated mesoporous silicas: structural, morphological, and adsorption properties. Langmuir 28, 11131–11141 (2012)
Cui, X., Bustin, R.M., Chikatamarla, L.: Adsorption-induced coal swelling and stress: implications for methane production and acid gas sequestration into coal seams. J. Geophys. Res 112, B10202 (2007)
Czwartos, J., Coasne, B., Gubbins, K.E., Hung, F.R., Sliwinska-Bartkowiak, M.: Freezing and melting of azeotropic mixtures confined in nanopores: experiment and molecular simulation. Mol. Phys 103, 3103–3113 (2005)
Day, S., Duffy, G., Sakurovs, R., Weir, S.: Effect of coal properties on CO2 sorption capacity under supercritical conditions. Int. J. Greenhouse Gas Control 2, 342–352 (2008)
Frenkel, D., Smit, B.: Understanding molecular simulation : from algorithms to applications, 2nd edn. Academic, San Diego; London (2002)
Gelb, L.D., Gubbins, K.E.: Pore size distributions in porous glasses: a computer simulation study. Langmuir 15, 305–308 (1999)
Gensterblum, Y., van Hemert, P., Billemont, P., Battistutta, E., Busch, A., Krooss, B.M., De Weireld, G., Wolf, Khaa: European inter-laboratory comparison of high pressure co2 sorption isotherms ii: natural coals. Int. J. Coal Geol. 84, 115–124 (2010)
Gensterblum, Y., van Hemert, P., Billemont, P., Busch, A., Charriére, D., Li, D., Krooss, B.M., de Weireld, G., Prinz, D., Wolf, K.H.A.A.: European inter-laboratory comparison of high pressure CO2 sorption isotherms. I: activated carbon. Carbon 47, 2958–2969 (2009)
Goodman, A.L., Busch, A., Bustin, R.M., Chikatamarla, L., Day, S., Duffy, G.J., Fitzgerald, J.E., Gasem, K.A.M., Gensterblum, Y., Hartman, C., Jing, C., Krooss, B.M., Mohammed, S., Pratt, T., Robinson Jr, R.L., Romanov, V., Sakurovs, R., Schroeder, K., White, C.M.: Inter-laboratory comparison II: cO2 isotherms measured on moisture-equilibrated Argonne premium coals at 55 °C and up to 15 MPa. Int. J. Coal Geol 72, 153–164 (2007)
Harris, J.G., Yung, K.H.: Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J Phy Chem 99, 12021–12024 (1995)
Heymans, N., Alban, B., Moreau, S., De Weireld, G.: Experimental and theoretical study of the adsorption of pure molecules and binary systems containing methane, carbon monoxide, carbon dioxide and nitrogen. Application to the syngas generation. Chem. Eng. Sci 66, 3850–3858 (2011)
Hildenbrand, A., Krooss, B., Busch, A., Gaschnitz, R.: Evolution of methane sorption capacity of coal seams as a function of burial history—a case study from the Campine Basin NE Belgium. Int. J. Coal Geol 66, 179–203 (2006)
Ho, L.N.: Perez Pellitero, J., Porcheron, F., Pellenq, R.J.M.: enhanced CO2 Solubility in Hybrid MCM-41: Molecular Simulations and Experiments. Langmuir 27, 8187–8197 (2011)
Jain, S.K., Pikunic, J.P., Pellenq, R.J.M., Gubbins, K.E.: Effects of activation on the structure and adsorption properties of a nanoporous carbon using molecular simulation. Adsorption 11, 355–360 (2005)
Jain, S.K., Pellenq, R.J.M., Pikunic, J.P., Gubbins, K.E.: Molecular modeling of porous carbons using the hybrid reverse Monte Carlo method. Langmuir 22(24), 9942–9948 (2006)
Jorge, M., Seaton, N.A.: Predicting adsorption of water/organic mixtures using molecular simulation. AIChE J. 49(8), 2059–2070 (2003). doi:10.1002/aic.690490815
Kaul, B.K.: A modern version of volumetric apparatus for measuring gas-solid equilibrium data. Ind. Eng. Chem. Res 26, 928–933 (1987)
Kunz, O., Wagner, W.: The GERG-2008 wide-range equation of state for natural gases and other mixtures: An expansion of GERG-2004. J. Chem. Eng, Data (2012)
Lewis, W.K., Gilliland, E.R., Chertow, B., Cadogan, W.P.: Adsorption equilibria hydrocarbon gas mixtures. Ind. Eng. Chem 42, 1319–1326 (1950)
Lippens, B.C., de Boer, J.H.: Studies on pore systems in catalysts: V. t method. J. Catal. 4, 319–323 (1965)
Mathews, J.P., van Duin, A., Chaffee, A.: The utility of coal molecular models. Fuel Proc. Technol 92, 718–728 (2011)
Mathews, J.P., Chaffee, A.: The molecular representations of coal—a review. Fuel 96, 1–14 (2012)
Mazzotti, M., Pini, R., Storti, G.: Enhanced coalbed methane recovery. J Supercrit. Fluids 47, 619–627 (2009). doi:10.1016/j.supflu.2008.08.013
Metz, B., Davidson, O., De Coninck, H., Loos, M., Meyer, L.: IPCC special report on carbon dioxide capture and storage. In. Intergovernmental Panel on Climate Change, Geneva (Switzerland). Working Group III, (2005)
Mikhail, R.S., Brunauer, S., Bodor, E.E.: Investigations of a complete pore structure analysis: I. Analysis of micropores. J. Colloid Interface Sci 26, 45–53 (1968)
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J 11, 121–127 (1965). doi:10.1002/aic.690110125
Nicholson, D., Gubbins, K.E.: Separation of carbon dioxide–methane mixtures by adsorption: effects of geometry and energetics on selectivity. J. Chem. Phys 104(20), 8126–8134 (1996)
Ottiger, S., Pini, R., Storti, G., Mazzotti, M., Bencini, R., Quattrocchi, F., Sardu, G., Deriu, G.: Adsorption of pure carbon dioxide and methane on dry coal from the Sulcis Coal Province (SW Sardinia, Italy). Environ. Prog 25, 355–364 (2006)
Setzmann, U., Wagner, W.: A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 20, 1061–1155 (1991)
Span, R., Lemmon, E.W., Jacobsen, R.T., Wagner, W., Yokozeki, A.: A reference equation of state for the thermodynamic properties of nitrogen for temperatures from 63.151 to 1000 k and pressures to 2200 Mpa. J. Phys. Chem. Ref. Data 29(6), 1361–1433 (2000)
Span, R., Wagner, W.: A New equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 25, 1509–1596 (1996)
Steele, W.A.: The physical interaction of gases with crystalline solids: I. Gas-solid energies and properties of isolated adsorbed atoms. Surf. Sci 36, 317–352 (1973)
Tambach, T.J., Mathews, J.P., van Bergen, F.: Molecular exchange of CH4 and CO2 in Coal: enhanced coalbed methane on a nanoscale. Energy Fuels 23, 4845–4847 (2009)
Tenney, C., Lastoskie, C.: Molecular simulation of carbon dioxide adsorption in chemically and structurally heterogeneous porous carbons. Environ. Prog 25, 343–354 (2006)
White, C.M., Smith, D.H., Jones, K.L., Goodman, A.L., Jikich, S.A., LaCount, R.B., DuBose, S.B., Ozdemir, E., Morsi, B.I., Schroeder, K.T.: Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery a review. Energy Fuels 19, 659–724 (2005)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Billemont, P., Coasne, B. & De Weireld, G. Adsorption of carbon dioxide-methane mixtures in porous carbons: effect of surface chemistry. Adsorption 20, 453–463 (2014). https://doi.org/10.1007/s10450-013-9570-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9570-z