Abstract
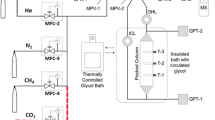
A dynamic column breakthrough (DCB) apparatus was used to measure the capacity and kinetics of CH4 and N2 adsorption on zeolite H+-mordenite at temperatures in the range 243.8–302.9 K and pressures up to 903 kPa. Equilibrium adsorption capacities of pure CH4 and pure N2 were determined by these dynamic experiments and Langmuir isotherm models were regressed to these pure fluid data over the ranges of temperature and pressure measured. A linear driving force-based model of adsorption in a fixed bed was developed to extract the mass transfer coefficients (MTCs) for CH4 and N2 from the pure gas experimental data. The MTCs determined from single adsorbate experiments were used to successfully predict the component breakthroughs for experiments with equimolar CH4 + N2 gas mixtures in the DCB apparatus. The MTC of CH4 on H+-mordenite at 902 kPa was 0.013 s−1 at 302.9 K and 0.004 s−1 at 243.6 K. The MTC of N2 on H+-mordenite at 902 kPa was 0.011 s−1 at 302.9 K and 0.005 s−1 at 243.5 K. The values of the MTCs measured for each gas at a constant feed gas flow rate were observed to increase in a linear trend with the inverse of pressure. However, the apparent MTCs obtained at the lowest pressures studied (≈105 kPa) were systematically below this linear trend, because of the slightly longer residence time of helium in the mass spectrometer used to monitor effluent composition. Nevertheless, the pure fluid dynamic breakthrough data at these lowest pressures could still be reasonably well described using MTC values estimated from the linear trend. Furthermore, the results of dynamic breakthrough experiments with mixtures were all reliably predicted using the capacity and MTC correlations developed for the pure fluids.








Similar content being viewed by others
References
Ackley, M.W., Yang, R.T.: Kinetic separation by pressure swing adsorption—method of characteristics model. AIChE J. 36(8), 1229–1238 (1990)
American Energies Pipeline, LLC: Improve Gas Quality-Nitrogen Rejection Unit. http://www.nitrogenrejectionunit.com (2011). Accessed 29 Oct 2010
Baker, R.W.: Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 41, 1393–1411 (2002)
Bevington, P.R., Robinson, D.K.: Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill, Boston (1992)
Bird, R.B., Stewart, W.E., Lightfoot, E.N.: Transport Phenomena, 2nd edn. Wiley International, New York (2002)
Casas, N., Schell, J., Pini, R., Mazzotti, M.: Fixed bed adsorption of CO2/H2 mixtures on activated carbon: experiments and modeling. Adsorption 18(2), 143–161 (2012). doi:10.1007/s10450-012-9389-z
Cavenati, S., Grande, C.A., Rodrigues, A.: Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 61, 3893–3906 (2006)
Churchill, S.W., Bernstein, M.: Correlating equation for forced-convection from gases and liquids to a circular-cylinder in cross-flow. J. Heat Transf. 99(2), 300–306 (1977)
Delgado, J.: A critical review of dispersion in packed beds. Heat Mass Transf. 42, 279–310 (2006). doi:10.1007/s00231-005-0019-0
Delgado, J.A., Uguina, M.A., Gomez, J.M., Ortega, L.: Adsorption equilibrium of carbon dioxide, methane and nitrogen onto Na- and H-mordenite at high pressures. Sep. Purif. Technol. 48(3), 223–228 (2006a). doi:10.1016/j.seppur.2005.07.027
Delgado, J.A., Uguina, M.A., Sotelo, J.L., Ruiz, B.: Modelling of the fixed-bed adsorption of methane/nitrogen mixtures on silicalite pellets. Sep. Purif. Technol. 50(2), 192–203 (2006b). doi:10.1016/j.seppur.2005.11.026
Guntuka, S., Farooq, S., Rajendran, A.: A- and B-Site substituted lanthanum cobaltite perovskite as high temperature oxygen sorbent. 2. Column dynamics study. Ind. Eng. Chem. Res. 47(1), 163–170 (2008). doi:10.1021/ie070860p
Hofman, P.S., Rufford, T.E., Chan, K.I., May, E.F.: A dynamic column breakthrough apparatus for adsorption capacity measurements with quantitative uncertainties. Adsorption 18(3–4), 251–263 (2012). doi:10.1007/s10450-012-9398-y
Jayaraman, A., Hernández-Maldonado, A.J., Yang, R.T., Chinn, D., Munson, C.L., Mohr, D.H.: Clinoptilolites for nitrogen/methane separation. Chem. Eng. Sci. 59, 2407–2417 (2004)
Jensen, N.K., Rufford, T.E., Watson, G.C.Y., Zhang, D., Chan, K.I., May, E.F.: Screening zeolites for gas separation applications involving methane, nitrogen, and carbon dioxide. J. Chem. Eng. Data 57(1), 106–113 (2012). doi:10.1021/je200817w
Kast, W.: Adsorption aus der gasphase—ingenieurwissenschaftliche Grundlagen und technische verfahren. VCH, Weinheim (1988)
Kidnay, A.J., Parrish, W.: Fundamentals of Natural Gas Processing. CRC Press, Boca Raton (2006)
Kunz, O., Klimeck, R., Wagner, W., Jaeschke, M.: The GERG-2004 Wide-Range Reference Equation of State for Natural Gases and Other Mixtures. GERG Technical Monograph. In. Fortschr.-Ber. VDI, VDI-Verlag, Düsseldorf (Germany), (2006)
Kuznicki, S.M., Bell, V.A., Petrovic, I., Blosser, P.W.: Separation of nitrogen from mixtures thereof with methane utilizing barium exchanged ETS-4. US Patent 5,989,316 (1999)
Lanfrey, P.Y., Kuzeljevic, Z.V., Dudukovic, M.P.: Tortuosity model for fixed beds randomly packed with identical particles. Chem. Eng. Sci. 65(5), 1891–1896 (2010). doi:10.1016/j.ces.2009.11.011
Lemmon, E.W., Huber, M.L., McLinden, M.O.: REFPROP—Reference Fluid Thermodynamic and Transport Properties. NIST Standard Reference Database 23 (2007)
Malbrunot, P., Vidal, D., Vermesse, J., Chahine, R., Bose, T.K.: Adsorbent helium density measurement and its effect on adsorption isotherms at high pressure. Langmuir 13, 539–544 (1997)
Malek, A., Farooq, S.: Determination of equilibrium isotherms using dynamic column breakthrough and constant flow equilibrium desorption. J. Chem. Eng. Data 41(1), 25–32 (1996)
Mitariten, M.: Nitrogen removal from natural gas with the molecular gate™ adsorption process. In: 88th Annual Convention of the Gas Processors Association 2009, San Antonio, TX, 8–11 March 2009, pp. 544–555. Gas Processors Association
Mohamed, M.M.: Heat capacities, phase transitions and structural properties of cation-exchanged H-mordenite zeolites. Thermochim. Acta 372(1–2), 75–83 (2001)
Mulgundmath, V.P., Jones, R.A., Tezel, F.H., Thibault, J.: Fixed bed adsorption for the removal of carbon dioxide from nitrogen: breakthrough behaviour and modelling for heat and mass transfer. Sep. Purif. Technol. 85, 17–27 (2012)
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11, 121–127 (1965)
Puértolas, B., Navarro, M.V., Lopez, J.M., Murillo, R., Mastral, A.M., Garcia, T.: Modelling the heat and mass transfer of propane onto a ZSM-5 zeolite. Sep. Purif. Technol. 86, 127–136 (2012)
Rajendran, A., Kariwala, V., Farooq, S.: Correction procedures for extra-column effects in dynamic column breakthrough experiments. Chem. Eng. Sci. 63(10), 2696–2706 (2008). doi:10.1016/j.ces.2008.02.023
Reid, R.C., Prausnitz, J.M., Poling, B.E.: The Properties of Gases and Liquids, 4th edn. McGraw-Hill, New York (1987)
Schiesser, W.E.: The Numerical Method of Lines. Academic Press, San Diego (1991)
Simo, M., Brown, C.J., Hlavacek, V.: Simulation of pressure swing adsorption in fuel ethanol production process. Comput. Chem. Eng. 32(7), 1635–1649 (2008). doi:10.1016/j.compchemeng.2007.07.011
Sircar, S.: Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 45(16), 5435–5448 (2006). doi:10.1021/ie051056a
Sircar, S., Hufton, J.R.: Why does the linear driving force model for adsorption kinetics work? Adsorption 6(2), 137–147 (2000). doi:10.1023/A:1008965317983
Sudibandriyo, M., Pan, Z., Fitzgerald, J.E., Robinson, R.L., Gasem, K.A.M.: Adsorption of methane, nitrogen, carbon dioxide, and their binary mixtures on dry activated carbon at 318.2 K and pressures up to 13.6 MPa. Langmuir 19(13), 5323–5331 (2003). doi:10.1021/la020976k
Valenzuela, D.P., Myers, A.L.: Adsorption Equilibrium Data Handbook. Advanced Reference Series. Prentice Hall, Englewood Cliffs (1989)
Warmuzinski, K., Tanczyk, M.: Multicomponent pressure swing adsorption. 1. Modelling of large-scale PSA installations. Chem. Eng. Process. 36(2), 89–99 (1997)
Watson, G., May, E.F., Graham, B.F., Trebble, M.A., Trengove, R.D., Chan, K.I.: Equilibrium adsorption measurement of pure nitrogen, carbon dioxide, and methane on a carbon molecular sieve at cryogenic temperatures and high pressures. J. Chem. Eng. Data 54, 2701–2707 (2009). doi:10.1021/je900224w
Yang, R.T.: Gas Separation by Adsorption Processes. Series on Chemical Engineering, vol. 1. World Scientific, Singapore (1997)
Acknowledgments
The research was funded by Chevron Energy Technology Company, the Western Australian Energy Research Alliance and the Australian Research Council (Project LP0776928). We thank Craig Grimm for helping to construct the apparatus, as well as David Zhang for his contribution to the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saleman, T.L.H., Watson, G.C.Y., Rufford, T.E. et al. Capacity and kinetic measurements of methane and nitrogen adsorption on H+-mordenite at 243–303 K and pressures to 900 kPa using a dynamic column breakthrough apparatus. Adsorption 19, 1165–1180 (2013). https://doi.org/10.1007/s10450-013-9546-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9546-z