Abstract
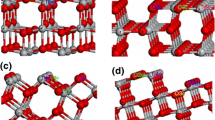
Microscopic structures of Zn(II) adsorbed on anatase TiO2 surface with different densities were studied using extended X-ray absorption fine structure (EXAFS) spectroscopy and density functional theory (DFT) calculation. Quantitative analysis of the EXAFS spectra showed that microscopic structures of Zn(II) were fourfold coordinated complexes, and different microscopic structures were present of the solid surface. Three modes of corner–corner/sharing-corner/sharing-edge adsorptions on anatase (101) face cluster were calculated by the DFT method. The results from DFT method were consistent with the EXAFS fittings. The optimized Zn–O average distance of the Zn–O tetrahedron was determined as about 2.00 Å. The Zn–Ti distance was 3.69 Å for the corner–corner adsorption, 3.35 Å for the sharing-corner adsorption, and 3.02 Å for the sharing-edge adsorption. According to the adsorption energies calculated by the DFT method, the microscopic structure of corner–corner adsorption was less stable than those of the other adsorption modes. With the increasing adsorption density, the corner–corner adsorption mode would be enhanced more intensively than the other adsorption modes.





Similar content being viewed by others
References
Ankudinov, A.L., Ravel, B., Rehr, J.J., Conradson, S.D.: Real-space multiple scattering calculation of XANES. Phys. Rev. B 58, 7565–7576 (1998)
Barakat, M.A.: Adsorption behavior of copper and cyanide ions at TiO2–solution interface. J. Colloid Interface Sci. 291, 345–352 (2005)
Bochatay, L., Persson, P.: Metal ion coordination at the water–manganite (γ-MnOOH) interface: II. an EXAFS study of Zn(II). J. Colloid Interface Sci. 229, 593–599 (2000)
Brown Jr, G.E., Parks, G.A., O’Day, P.A.: In: Pattrick, R.A.D., Vaughan, D.J. (eds.) Mineral Surfaces, pp. 129–183. Chapman and Hall, London (1995)
Brummer, G., Tiller, K.G., Herms, U., Clayton, P.M.: Adsorption–desorption and/or precipitation–dissolution processes of Zn in soils. Geoderma 31, 337–354 (1983)
Charlet, L., Manceau, A.: Environmental analytical and physical chemistry series. In: Buffle, J., van Leeuwen, H.P. (eds.) Environmental Particles, vol. 2, pp. 117–164. Lewis Publishers, Boca Raton (1993)
Combes, J.M., Manceau, A., Calas, G., Bottero, J.Y.: Formation of ferric oxides from aqueous solutions: a polyhedral approach by X-ray absorption spectroscdpy: I. hydrolysis and formation of ferric gels. Geochim. Cosmochim. Acta 53, 583–594 (1989)
Coston, J.A., Fuller, C.C., Davis, J.A.: Pb2 + and Zn2 + adsorption by a natural Aluminum- and Iron-bearing surface coating on an aquifer sand. Geochim. Cosmochim. Acta 59, 3535–3547 (1995)
Frich, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr, J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cm, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challa-combe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision C.02. Gaussian, Wallingford (2004)
Kiekens, L.: In: Alloway, B.J. (ed.) Heavy Metals in Soils, p. 284. Blackie, London (1990)
Kim, M.S., Chung, J.G.: A study on the adsorption characteristics of orthophosphates on rutile-type titanium dioxide in aqueous solutions. J. Colloid Interface Sci. 233, 31–37 (2001)
Ladeira, A.C.Q., Ciminell, V.S.T., Duarte, H.A., Alves, M.C.M., Ramos, A.Y.: Mechanism of anion retention from EXAFS and density functional calculations: arsenic (V) adsorbed on gibbsite. Geochim. Cosmochim. Acta 65, 1211–1217 (2001)
Lee, S., Anderson, P.R., Bunker, G.B., Karanfil, C.: EXAFS study of Zn sorption mechanisms on montmorillonite. Environ. Sci. Technol. 385, 426–5432 (2004)
Li, X., Pan, G., Qin, Y., Hu, T., Xie, Y., Chen, H.: EXAFS studies on adsorption microscopic structures of Zn at manganite-water interface. J. High Energy Phys. Nucl. Phys. 27, 23–27 (2003). Chinese edition
Li, X., Pan, G., Qin, Y., Hu, T., Wu, Z., Xie, Y.: EXAFS studies on adsorption–desorption reversibility at manganese oxide–water interfaces: II. reversible adsorption of Zn on δ-MnO2. J. Colloid Interface Sci. 271, 35–40 (2004)
Li, W., Pan, G., Zhang, M.Y., Zhao, D.Y., Yang, Y.H., Chen, H., He, G.Z.: EXAFS studies on adsorption irreversibility of Zn(II) on TiO2: temperature dependence. J. Colloid Interface Sci. 319, 385–391 (2008)
Muller, B., Sigg, L.: Interaction of trace metals with natural particle surfaces: comparison between adsorption experiments and field measurements. Aquat. Sci. 52, 75–92 (1990)
Nachtegaal, M., Sparks, D.L.: Effect of iron oxide coatings on zinc sorption mechanisms at the clay-mineral/water interface. J. Colloid Interface Sci. 276, 13–23 (2004)
Pan, G., Qin, Y., Li, X., Hu, T., Wu, Z., Xie, Y.: EXAFS studies on adsorption–desorption reversibility at manganese oxides–water interfaces: I. irreversible adsorption of Zn onto manganite (γ-MnOOH). J. Colloid Interface Sci. 271, 28–34 (2004)
Papelis, C., Brown Jr, G.E., Parks, G.A., Leckie, J.O.: X-ray absorption spectroscopic studies of cadmium and selenite adsorption on aluminum oxides. Langmuir 11, 2041–2048 (1995)
Ragnarsdottir, K.V., Collins, C.R.: The mechanism of cadmium surface complexation on iron oxyhydroxide minerals. Geochim. Cosmochim. Acta 63, 2971–2987 (1999)
Ravel, B.: ATOMS, crystallography for the X-ray absorption spectroscopist. J. Synchroton Radiat. 8, 314–316 (2001)
Ressler, T.: WinXAS, a program for X-ray absorption spectroscopy data analysis under MS-windows. J. Synchrotron Radiat. 5, 118–122 (1998)
Roe, A.L., Hayes, K.F., Chisholm-Brause, C.J., Brown Jr, G.E., Parks, G.A., Hodgson, K.O., Leckie, J.O.: In-situ X-ray absorption study of lead ion surface complexes at the goethite-water interface. Langmuir 7, 367–373 (1991)
Scheidigger, A.M., Lamble, G.M., Sparks, D.L.: Spectroscopic evidence for the formation of mixed-cation hydroxide phases upon metal sorption on clays and Aluminum Oxides. J. Colloid Interface Sci. 186, 118–128 (1997)
Shock, E.L., Koretsky, C.M.: Etal-organic complexes in geochemical processes: calculation of standard partial molal thermodynamic properties of aqueous acetate complexes at high pressures and temperatures. Geochim. Cosmochim. Acta 57, 4899–4922 (1993)
Spadini, L., Manceau, A., Schindler, P.W., Charlet, L.: Structure and stability of Cd2+ surface complexes on ferric oxides: 1. Results from EXAFS spectroscopy. J. Colloid Interface Sci. 168, 73–86 (1994)
Tessier, A., Carignan, R., Dubruel, B., Rapin, F.: Partitioning of Zn between the water column and the oxic sediments in lakes. Geochim. Cosmochim. Acta 53, 1511–1522 (1989)
Towle, S.N., Brown, G.E., Parks, G.A.: Sorption of Co(II) on metal oxide surfaces: I. Identification of specific binding sites of Co(II) on (110) and (001) surfaces of TiO2 (rutile) by Grazing-incidence XAFS spectroscopy. J. Colloid Interface Sci. 217, 299–311 (1999)
Trainor, T.P., Brown, G.E., Parks, G.A.: Adsorption and precipitation of aqueous Zn(II) on alumina powders. J. Colloid Interface Sci. 231, 359–372 (2000)
Trivedi, P., Axe, L., Tyson, T.A.: An analysis of Zn sorption to amorphous versus crystalline iron oxides using XAS. J. Colloid Interface Sci. 244, 230–238 (2001)
Ulrike, D.: The surface science of TiO2. Surf. Sci. Rep. 48, 53–229 (2003)
Vittadini, A., Selloni, A., Rotzinger, F.P., Gratzel, M.: Structure and energetics of water adsorbed at TiO2 anatase (101) and (001) surfaces. J. Phys. Chem. C 104, 1300–1306 (2000)
Vymazal, J.: Occurrence and chemistry of Zn in freshwaters—its toxicity and bioaccumulation with respect to algae: a review. Part 1: occurrence and chemistry of Zn. Acta Hydrochem. Hydrobiol. 13, 627–654 (1985)
Waychunas, G.A., Rea, B.A., Fuller, C.C., Davis, J.A.: Surface chemistry of ferrihydrite:Part 1.EXAFS study of geometry of coprecipitated and adsorbed arsenate. Geochim. Cosmochim. Acta 57, 2251–2269 (1993)
Waychunas, G.A., Davis, J.A., Fuller, C.C.: Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH. Geochim. Cosmochim. Acta 59, 3655–3661 (1995)
Zhang, P.C., Brady, P.V., Arthur, S.E., Zhou, W.Q.: Adsorption of barium (II) on montmorillonite: an EXAFS study. Colloids Surf. A 190, 239–249 (2001)
Zhu, M., Pan, G.: Quantum chemical studies of mononuclear zinc species of hydration and hydrolysis. J. Phys. Chem. A 109, 7648–7652 (2005)
Acknowledgments
The authors thank the institute of high energy physics Chinese academy of sciences for the EXAFS spectrum unscrambling and soft support, and thank the supercomputing center of Chinese academy of sciences for the DFT soft. This work was financially supported by NNSF of China (Grant No. 21107054).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Chen, H. & Ye, C. EXAFS and DFT studies of microscopic structure with different density upon Zn(II) adsorption on anatase TiO2 . Adsorption 19, 1019–1025 (2013). https://doi.org/10.1007/s10450-013-9510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9510-y